A kind of homogenine hydrobromide compound and preparation thereof
A technology of clathrin hydrobromide and compound, which is applied in the field of medicine, can solve the problems of insignificant change of pH of solution, unstable and falling under strong light irradiation, and achieves good stability, safe and reliable clinical application, and improved stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
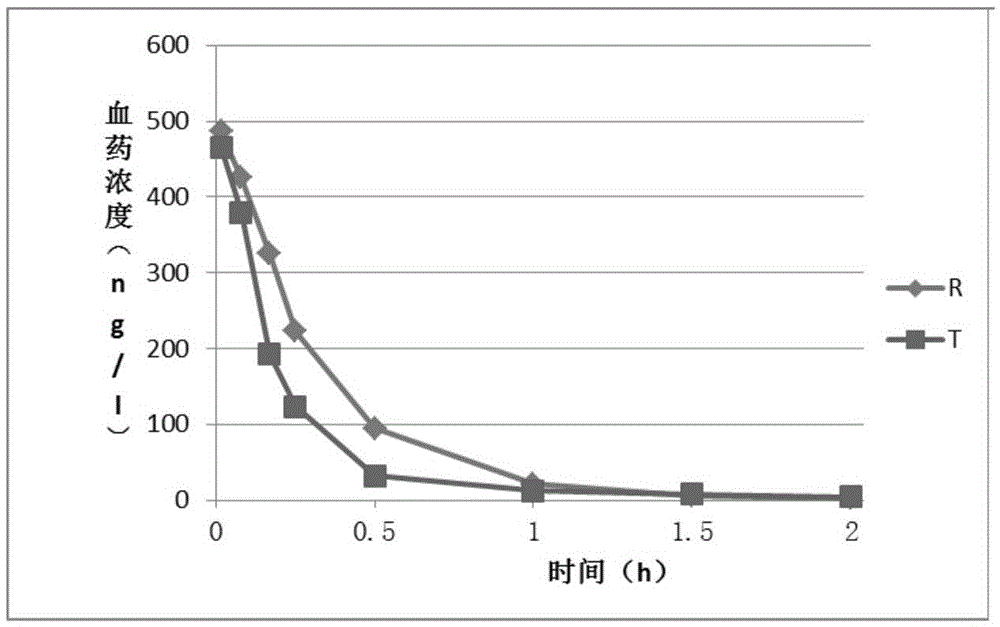
Image
Examples
Embodiment 1
[0048] 1. Grind the crude homogenin hydrobromide, pass it through a 200-mesh sieve, and then add it to anhydrous methanol at 45°C to prepare a saturated solution of homogenin hydrobromide;
[0049]2. While stirring, add water: dimethyl sulfoxide: ethyl acetate mixed solution with a volume ratio of 3:1:1, and lower the temperature to 10°C at the same time; the stirring speed is 360 rpm; the weight of the mixed solvent is the weight of the methanol solution 8 times of , the adding speed is 20 ml / min;
[0050] 3. After adding the mixed solution, let it stand still, and continue to cool down to 2°C at a cooling rate of 1.5°C / hour; grow the crystal for 4 hours, wash, and dry to obtain homogenin hydrobromide dihydrate.
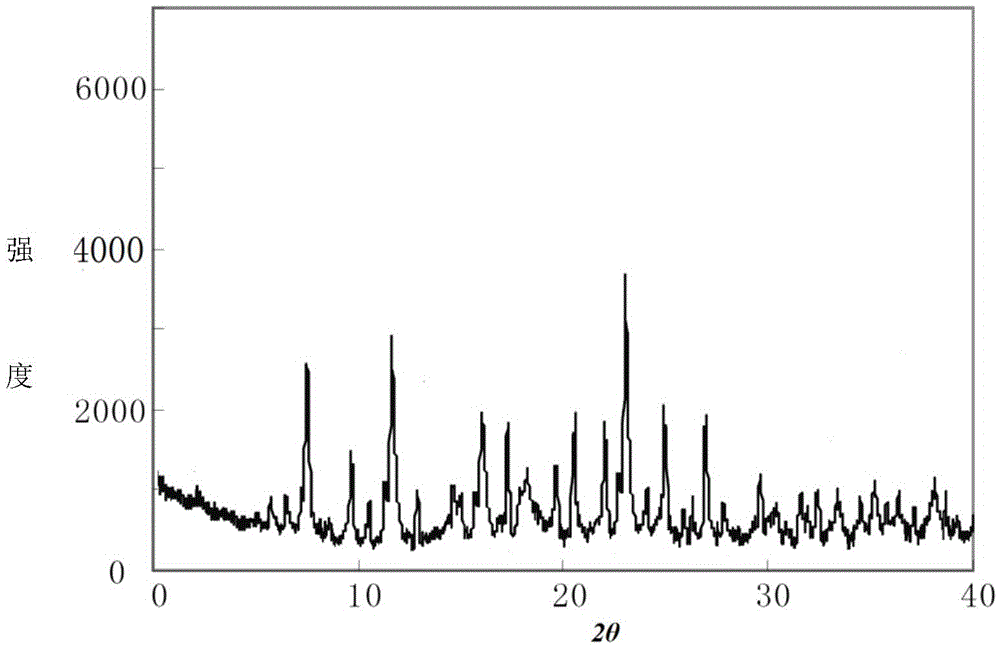
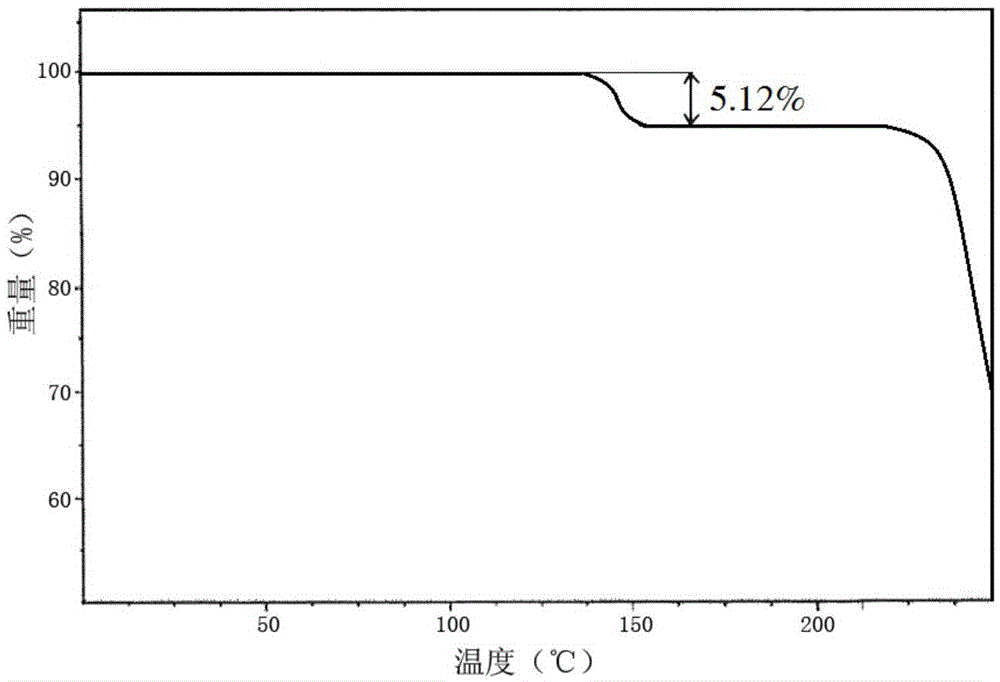
[0051] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.97%, and the yield is 94.7%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 1 As shown, the thermogravimetric...
Embodiment 2
[0053] 1. Grind the crude homogenin hydrobromide, pass through a 300-mesh sieve, and then add it to anhydrous methanol at 40°C to prepare a saturated solution of homogenin hydrobromide;
[0054] 2. While stirring, add water: dimethyl sulfoxide: ethyl acetate mixed solution with a volume ratio of 2:1:2, and lower the temperature to 20°C at the same time; the stirring speed is 720 rpm; the weight of the mixed solvent is the weight of the methanol solution 12 times of , the adding speed is 40 ml / min;
[0055] 3. After adding the mixed solution, let it stand still, and continue to cool down to 4°C at a cooling rate of 1.5-3°C / hour; grow crystals for 3 hours, wash, and dry to obtain homogenin hydrobromide dihydrate.
[0056] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.97%, and the yield is 95.1%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 1 As shown, the thermogravimetric a...
Embodiment 3
[0058] 1. Grind the crude homogenin hydrobromide, pass through a 300-mesh sieve, and then add it to anhydrous methanol at 42°C to prepare a saturated solution of homogenin hydrobromide;
[0059] 2. While stirring, add water: dimethyl sulfoxide: ethyl acetate mixed solution with a volume ratio of 3:1:3, and lower the temperature to 15°C at the same time; the stirring speed is 480 rpm; the weight of the mixed solvent is the weight of the methanol solution 10 times of , the adding speed is 30 ml / min;
[0060] 3. After adding the mixed solution, let it stand still, continue to cool down to 2°C, and the cooling rate is 2°C / hour; grow crystals for 4 hours, wash, and dry to obtain homouginin hydrobromide dihydrate.
[0061] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.97%, and the yield is 94.3%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 1 As shown, the thermogravimetric anal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com