Novel chemical skin penetration enhancer containing cholic acid group
A technology of absorption enhancer and enhancer, applied in the field of medicine, can solve problems such as toxic and side effects reducing application value, and achieve the effects of increasing solubility, excellent penetration promoting effect, and enhancing fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
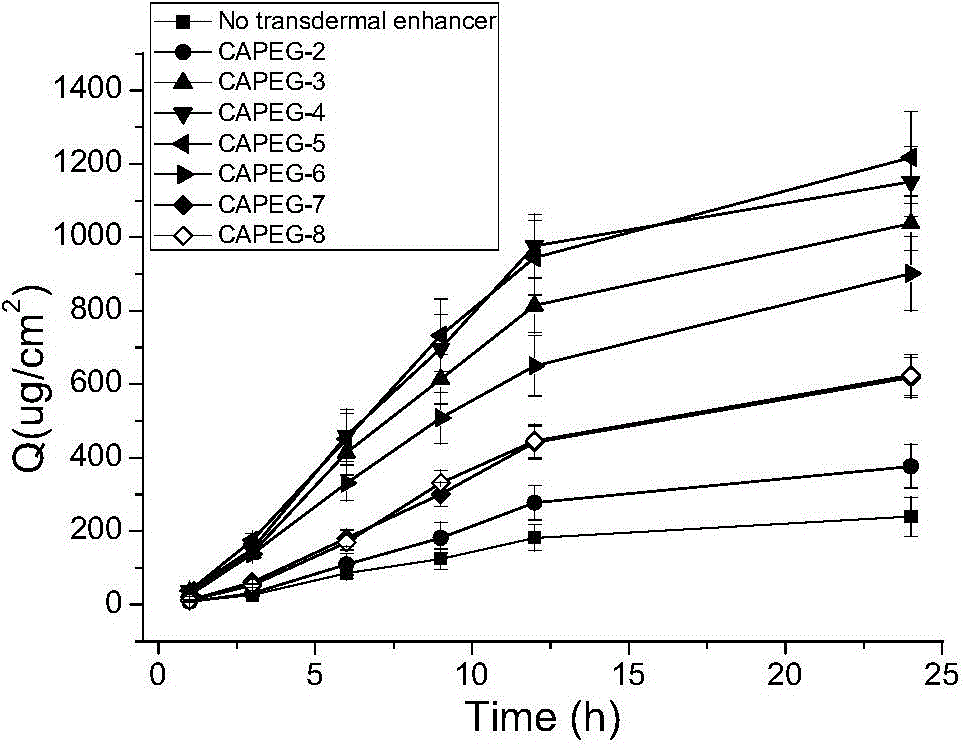
Image
Examples
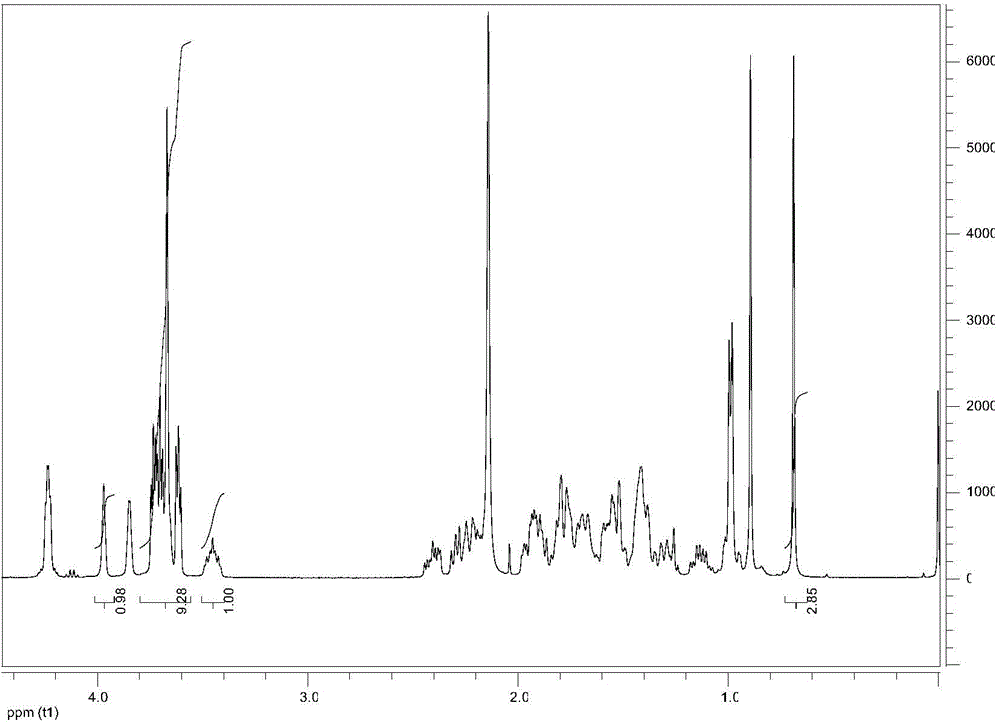
Embodiment 1
[0036] Diethylene glycol cholate (CAPEG-2) is prepared through the following steps:
[0037] (1) In a 250mL round bottom flask, dissolve 2g (4.9mmol) cholic acid and 0.6g (6.0mmol) diethylene glycol in 50mL dichloromethane (DCM), and add 0.05g (0.4mmol) Catalyst 4-lutidine (DMAP);
[0038] (2) Dissolve 1.24g (6.0mmol) N,N-dicyclohexyldiimine (DCC) in a mixed solvent of 10mL dichloromethane and acetonitrile (dichloromethane / acetonitrile=1 / 1), and stir state, the solution was dropped into the system of step (1), and reacted at room temperature for 18h;
[0039] (3) After the reaction is over, add 60mL of water to the system, then transfer it to a separatory funnel, let it stand until the layers are separated, remove the water layer, and wash the organic solution layer three times with 20mL distilled water and three times with 20mL saturated sodium bicarbonate solution , washed three times with 20 mL of saturated brine, collected the final organic phase, and dried with anhydrou...
Embodiment 2
[0042] Triethylene glycol cholate (CAPEG-3) is prepared through the following steps:
[0043] (1) In a 250mL round bottom flask, dissolve 2g (4.9mmol) cholic acid and 0.9g (6.0mmol) triethylene glycol in 30mL dichloromethane (DCM), and add 0.05g (0.4mmol) Catalyst 4-lutidine (DMAP);
[0044] (2) Dissolve 1.24g (6.0mmol) N,N-dicyclohexyldiimine (DCC) in a mixed solvent of 10mL dichloromethane and acetonitrile (dichloromethane / acetonitrile=1 / 1), and stir state, the solution was dropped into the system of step (1), and reacted at room temperature for 18h;
[0045] (3) After the reaction is over, add 60mL of water to the system, then transfer it to a separatory funnel, let it stand until the layers are separated, remove the water layer, and wash the organic solution layer three times with 20mL distilled water and three times with 20mL saturated sodium bicarbonate solution , washed three times with 20 mL of saturated brine, collected the final organic phase, and dried with anhydr...
Embodiment 3
[0049] Triethylene glycol cholate (CAPEG-4) is prepared through the following steps:
[0050] (1) In a 250mL round bottom flask, dissolve 2g (4.9mmol) cholic acid and 1.16g (6.0mmol) tetraethylene glycol in 50mL dichloromethane (DCM), and add 0.05g (0.4mmol) Catalyst 4-lutidine (DMAP);
[0051] (2) Dissolve 1.24g (6.0mmol) N,N-dicyclohexyldiimine (DCC) in a mixed solvent of 10mL dichloromethane and acetonitrile (dichloromethane / acetonitrile=1 / 1), and stir state, the solution was dropped into the system of step (1), and reacted at room temperature for 18h;
[0052] (3) After the reaction is over, add 60mL of water to the system, then transfer it to a separatory funnel, let it stand until the layers are separated, remove the water layer, and wash the organic solution layer three times with 20mL distilled water and three times with 20mL saturated sodium bicarbonate solution , washed three times with 20 mL of saturated brine, collected the final organic phase, and dried with anh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com