Organic titanium catalyst for exchange reaction of dimethyl carbonate and phenol ester
A technology of dimethyl carbonate and diphenyl carbonate, applied in the field of organic titanium catalysts, can solve problems such as the inability to reuse heterogeneous catalysts, and achieve the effects of easy separation and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
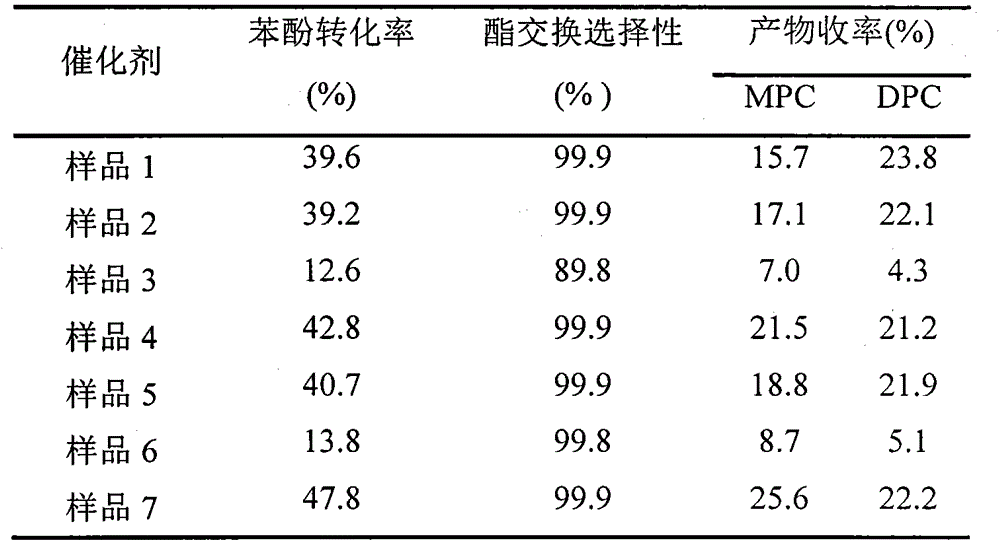
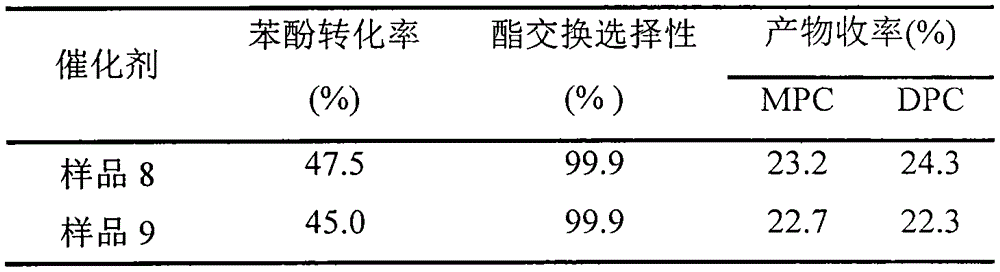
[0021] Using butyl titanate (0.05mol) and acetic anhydride as raw materials, 50ml of cyclohexane as solvent, the molar ratio of titanium to water is 1, and the molar ratio of titanium to acetic acid is 0.2, the reactor is reacted at 90°C, and after calcination at 200°C Catalyst sample 1 was obtained.
Embodiment 2
[0023] Using butyl titanate (0.05mol) and trifluoroacetic acid as raw materials, cyclohexane as the solvent, the molar ratio of titanium to water is 1, and the molar ratio of titanium to trifluoroacetic acid is 0.2. Catalyst sample 2 was obtained after calcination.
Embodiment 3
[0025] Using titanyl sulfate (0.05mol) and acetic anhydride as raw materials, cyclohexane as the solvent, the molar ratio of titanium to water is 1, and the molar ratio of titanium to acetic anhydride is 0.2, the reactor is reacted at 90°C and roasted at 200°C to obtain Catalyst Sample 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com