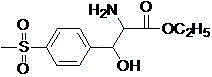
Preparation method of p-methyl sulfone phenyl ethyl serinate
A technology for methylsulfonyl phenylserine ethyl ester and methylsulfonyl phenylserine ethyl ester, which is applied in the field of preparation of p-methylsulfonyl phenylserine ethyl ester, can solve problems such as failure to meet requirements, cancellation of production qualifications, and the like, and achieve the effect of solving environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Synthesis of p-Methylsulfonyl Phenylserine
[0016] Dissolve 20g of copper p-methylsulfonylphenylserine in sodium hydroxide solution (6g NaOH dissolved in 84mL water), add Na 2 S solution (12.1g Na 2 S was dissolved in 17 mL of water), stirred mechanically for 4 hours, then added 8 g of activated carbon to the mixture, stirred mechanically for 1 hour, and filtered with suction. The filtrate was transferred to a 250mL three-necked flask, concentrated hydrochloric acid was added dropwise to adjust the pH to 3, the crystals were concentrated, and filtered with suction to obtain 13.39g of p-methylsulfonylphenylserine with a yield of 75.4%.
Embodiment 2
[0018] Synthesis of p-Methylsulfonyl Phenylserine
[0019] Dissolve 20g of copper p-methylsulfonylphenylserine in sodium hydroxide solution (6g NaOH dissolved in 84mL water), add Na 2 S solution (12.1g Na 2 S was dissolved in 17 mL of water), stirred mechanically for 4 hours, then added 8 g of activated carbon to the mixture, stirred mechanically for 1 hour, and filtered with suction. The filtrate was transferred to a 250 mL three-necked flask, concentrated hydrochloric acid was added dropwise to adjust the pH to 4, the crystals were concentrated, and filtered with suction to obtain 13.5 g of p-methylsulfonyl phenylserine with a yield of 76%.
Embodiment 3
[0021] Synthesis of p-Methylsulfonyl Phenylserine
[0022] Dissolve 20g of copper p-methylsulfonylphenylserine in sodium hydroxide solution (6g NaOH dissolved in 84mL water), add Na 2 S solution (12.1g Na 2 S was dissolved in 17 mL of water), stirred mechanically for 4 hours, then added 8 g of activated carbon to the mixture, stirred mechanically for 1 hour, and filtered with suction. The filtrate was transferred to a 250 mL three-necked flask, concentrated hydrochloric acid was added dropwise to adjust the pH to 5, the crystals were concentrated, and filtered with suction to obtain 13.85 g of p-methylsulfonyl phenylserine with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com