A kind of preparation method of ferrocene diphosphine ligand
A bisphosphine ligand and ferrocene technology, which is applied in the synthesis of organic phosphine compounds and ferrocene bisphosphine compounds, can solve the problems of high production equipment requirements, low reaction yield, and difficult reaction control, and achieve Optimize the separation and purification process, facilitate industrial production, and change the effect of harsh requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
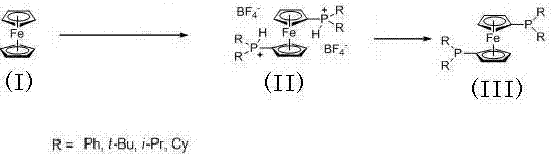
[0015] Example 1 Synthesis of 1,1'-bis(diphenylphosphino)ferrocene
[0016] Under the protection of argon, ferrocene (1 mol, 186 g), 1 L 1,2-dichloroethane and diphenylphosphine oxide (2 mol, 405 g) were added to the dry reactor at 0 °C Add dropwise 47% mass percent boron trifluoride ether solution (5 mol, 1.52 kg) to the system under the same conditions. After the dropwise addition, raise the temperature to 60°C for 10 h, then cool down to 0°C and add water dropwise to the system for hydrolysis. Then separate the liquids, dry the organic layer with anhydrous magnesium sulfate, filter, and distill off the solvent under reduced pressure to obtain a yellow solid, which can be recrystallized from dichloromethane and n-hexane to obtain 1,1'-bis(diphenylphosphine)dicene Tetrafluoroborate of iron 686 g ( 31 PNMR (400 MHz, CDCl 3 ), δ: 4.0 ppm); add 2 L methanol to the above tetrafluoroborate, heat to reflux for 8 h, then cool and crystallize under the protection of argon, filter a...
Embodiment 2
[0017] Example 2 Synthesis of 1,1'-bis(di-tert-butylphosphino)ferrocene
[0018] Under the protection of argon, ferrocene (1 mol, 186 g), 1 L of 1,2-dichloroethane and di-tert-butylphosphine oxide (4 mol, 648 g) were added to the dry reactor. At 10°C, a 47% by mass boron trifluoride ether solution (8 mol, 2.4 kg) was added dropwise to the system. After the dropwise addition, the temperature was raised to 80°C for 10 h, and then the temperature was lowered to 0°C, and water was added dropwise to the system. Hydrolyze, then separate the liquids, dry the organic layer with anhydrous magnesium sulfate, filter, and distill off the solvent under reduced pressure to obtain a yellow solid, which can be recrystallized from dichloromethane and n-hexane to obtain 1,1'-bis(di-tert-butylphosphine ) Tetrafluoroborate 618g of ferrocene ( 31 P NMR (400 MHz, CDCl 3 ), δ: 39.9 ppm); add 2 L methanol to the above tetrafluoroborate, heat to reflux for 12 h, then cool and crystallize under the p...
Embodiment 3
[0019] Example 3 Synthesis of 1,1'-bis(diisopropylphosphino)ferrocene
[0020] Under the protection of argon, add ferrocene (1 mol, 186 g) and diisopropylphosphine oxide (4 mol, 536 g) into the dry reactor (synthesized according to the method of document Organometallics, 2009, 28, 6383~6401 ) and 1 L of 1,2-dichloroethane, add 47% by mass boron trifluoride ether solution (8 mol, 2.4 kg) dropwise to the system at 10°C, and then raise the temperature to 70°C to react 12 h, then lower the temperature to 0°C, add water dropwise to the system, hydrolyze, then separate the liquids, dry the organic layer with anhydrous magnesium sulfate, filter, and distill off the solvent under reduced pressure to obtain a yellow solid, which can be recrystallized from dichloromethane and n-hexane Obtain 552 g of tetrafluoroborate of 1,1'-bis(diisopropylphosphino)ferrocene; add 1 L of methanol to the above tetrafluoroborate, heat to reflux for 12 h, and then cool under the protection of argon Cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com