Preparation method of draxxin
A technology of tyramectin and a synthesis method, applied in the field of preparation of tyramectin, can solve the problems of harsh reaction conditions, high price of di-tert-butyl dicarbonate, high cost and safety, etc., and achieves rapid reaction and comprehensive The effect of improving yield and shortening operation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
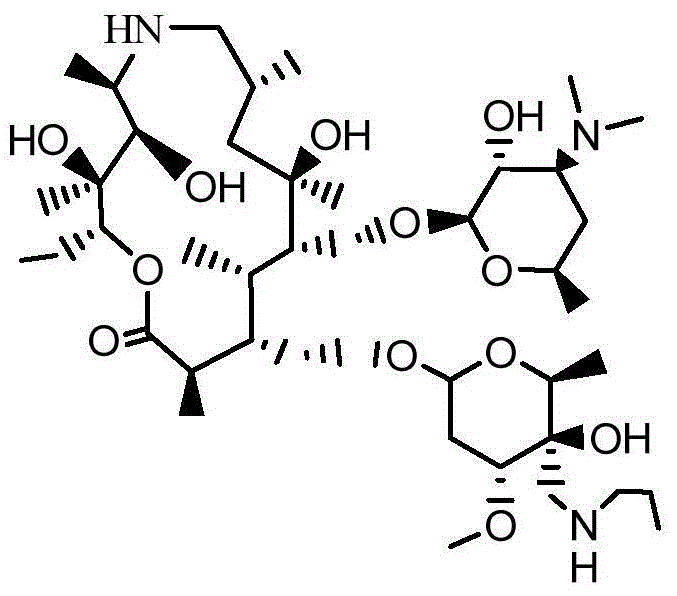
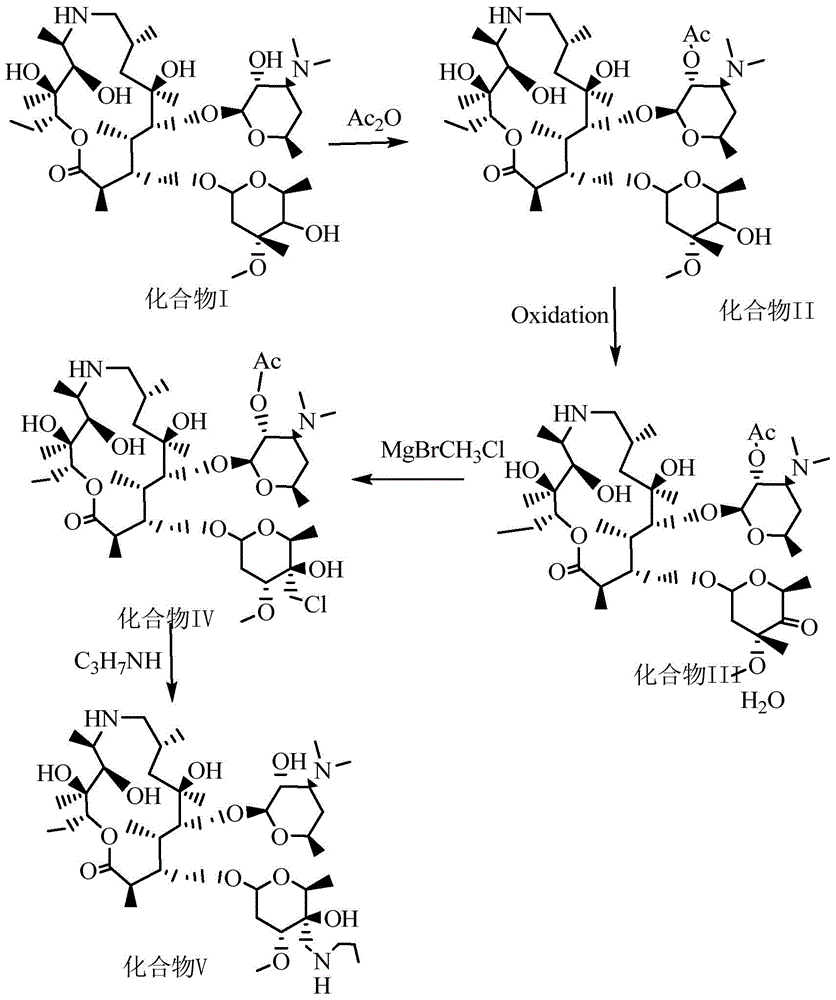
[0031] ((2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-Dideoxy-3-C-methyl-3-O-methyl--α -L-nucleo-hexapyranosyl]-oxy]-2-ethyl-3,4,10-trihydroxy acid-3,5,8,10,12,14-hexamethyl-11-[[ 3,4,6-Tideoxy-3-(dimethylamino)-2-O-acetyl-β-D-wood-hexapyranosyl]oxy)-1-oxa-6-azacyclodeca Pentaxane (15-membered macrocycle) (attached Figure II Preparation of compound II):
[0032] Add 500 ml of dichloromethane to a 1000 mL three-necked reaction flask, raise the temperature to 40° C., add 50 g of desmethyl azithromycin, start stirring to dissolve. Then add 6.9 g of triethylamine and stir evenly. Then, 6.9 g of acetic anhydride was slowly added, and the reaction was stirred for 3 hours to obtain a reaction liquid. After the reaction is over, add 80ml of water to the above reaction solution, add sodium hydroxide to adjust the pH value to 9, stir for 10 minutes, stand for phase separation, wash the water phase with dichloromethane, combine the organic phases, and add to the organic phase 80mL sat...
Embodiment 2
[0034] ((2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-Dideoxy-3-C-methyl-3-O-methyl--α -L-nucleo-hexapyranosyl]-oxy]-2-ethyl-3,4,10-trihydroxy acid-3,5,8,10,12,14-hexamethyl-11-[[ 3,4,6-Tideoxy-3-(dimethylamino)-2-O-acetyl-β-D-wood-hexapyranosyl]oxy)-1-oxa-6-azacyclodeca Preparation of pentane (15-membered macrocycle) (compound II)
[0035] Add 500ml of acetone to a 1000mL three-necked reaction flask, heat to 30°C, add 50g of desmethylazithromycin, start stirring to dissolve. Then add 9.9 g of diethylamine and stir evenly. Then slowly add 20.8 g of acetic anhydride, stir and react for 1 hour to obtain a reaction liquid. After the reaction is over, add 80ml of water to the above reaction solution, add sodium hydroxide to adjust the pH to 9.5, stir for 10 minutes, stand for phase separation, wash the water phase with dichloromethane, combine the organic phases, and add to the organic phase 80mL saturated NaCl solution, washed, phase separation, take the organic phase, add an ap...
Embodiment 3
[0037] ((2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-Dideoxy-3-C-methyl-3-O-methyl--α -L-nucleo-hexapyranosyl]-oxy]-2-ethyl-3,4,10-trihydroxy acid-3,5,8,10,12,14-hexamethyl-11-[[ 3,4,6-Tideoxy-3-(dimethylamino)-2-O-acetyl-β-D-wood-hexapyranosyl]oxy)-1-oxa-6-azacyclodeca Preparation of pentane (15-membered macrocycle) (compound II)
[0038] Add 500 ml of trichloromethane to a 1000 mL three-necked reaction flask, raise the temperature to 50° C., add 50 g of desmethyl azithromycin, start stirring and dissolve. Then add 26.8 g of pyridine and stir evenly. Then slowly add 234 g of acetic anhydride, stir and react for 5 hours to obtain a reaction liquid. After the reaction is over, add 80ml of water to the above reaction solution, add sodium hydroxide to adjust the pH to 10, stir for 10 minutes, stand for phase separation, wash the water phase with dichloromethane, combine the organic phases, and add to the organic phase 80mL saturated NaCl solution, washed, phase separation, take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com