Modified magnalium composite oxide catalyst for acetone condensation as well as preparation method and application thereof
A technology of composite oxides and catalysts, applied in the direction of metal/metal oxide/metal hydroxide catalysts, carbon-based compound preparation, organic compound preparation, etc., can solve the problems of isophorone to be improved and achieve orderly Good degree of crystallinity, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Alkaline solution preparation: Weigh 35.4g NaOH and 20.1g NaOH respectively 2 CO 3 Put it into a beaker, add an appropriate amount of double distilled water, stir to make NaOH, Na 2 CO 3 Dissolve completely and prepare 350ml alkali solution.
[0038] 2. Salt solution preparation: weigh 72.1g Mg(NO 3 ) 2 ·6H 2 O, 52.8g Al(NO 3 ) 3 9H 2 O, 24.5gCe(NO 3 ) 3 ·6H 2 O and 36.6g La(NO 3 ) 3 ·6H 2 O crystals were put into a beaker, an appropriate amount of twice distilled water was added, and stirred to completely dissolve the crystals, and a 350ml salt solution was prepared.
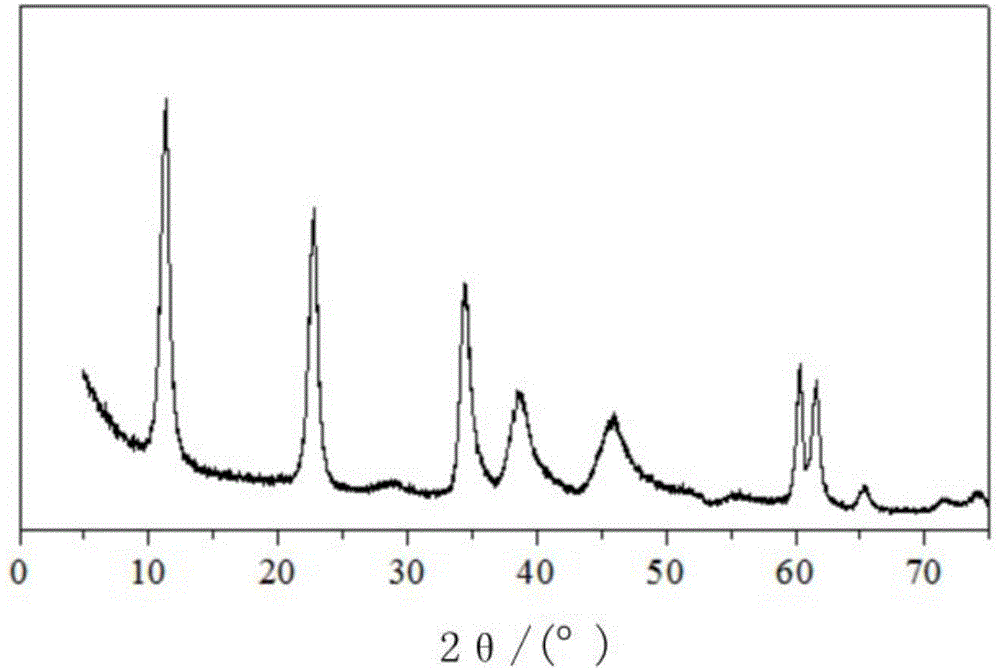
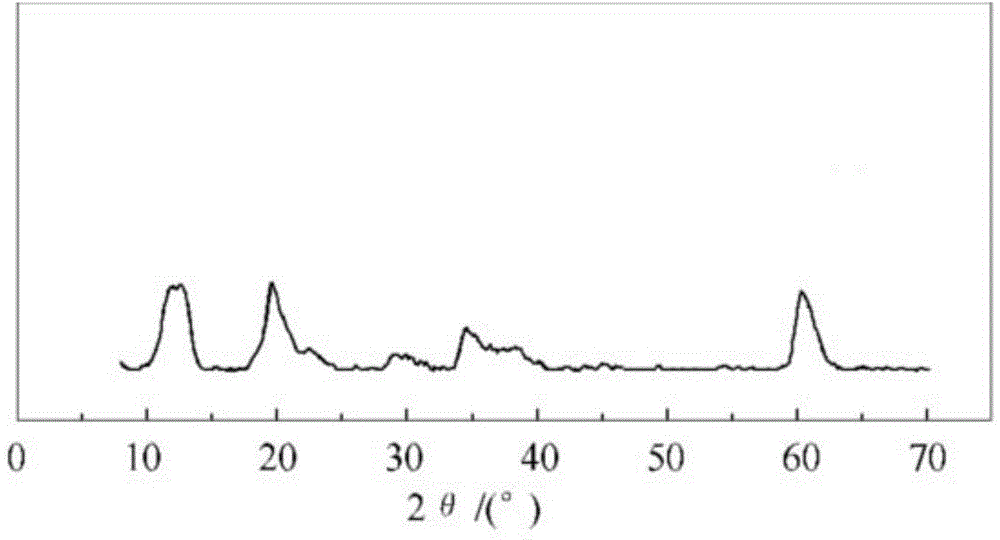
[0039] 3. Hydrotalcite preparation: put the flask into a constant temperature water bath and fix it on an iron stand, and add 300ml of deionized water into the flask. Adjust the temperature of the constant temperature water bath to 60°C, and start the stirrer. The salt solution and the alkali solution are fed into the flask in parallel by adopting a double-drop feeding method, and the ...
Embodiment 2
[0042] 1. Alkaline solution preparation: Weigh 34.8g NaOH and 20.1g NaOH respectively 2 CO 2 Put it into a beaker, add an appropriate amount of double distilled water, stir to make NaOH, Na 2 CO 3 Dissolve completely and prepare 350ml alkali solution.
[0043] 2. Salt solution preparation: weigh 72.4g Mg(NO 3 ) 2 ·6H 2 O, 52.3g Al(NO 3 ) 3 9H 2 O, 21.7g Y(NO 3 ) 3 ·6H 2 O and 24.2g Ce(NO 3 ) 3 ·6H 2 O crystals were put into a beaker, an appropriate amount of twice distilled water was added, and stirred to completely dissolve the crystals, and a 350ml salt solution was prepared.
[0044] 3. Hydrotalcite preparation: put the flask into a constant temperature water bath and fix it on an iron stand, and add 300ml of deionized water into the flask. Adjust the temperature of the constant temperature water bath to 75°C, and start the stirrer. The salt solution and the alkali solution are fed into the flask in parallel by adopting a double-drop feeding method, and the ...
Embodiment 3
[0048] 1. Alkaline solution preparation: Weigh 36.4g NaOH and 20.7g NaOH respectively 2 CO 3 Put it into a beaker, add an appropriate amount of double distilled water, stir to make NaOH, Na 2 CO 3 Dissolve completely and prepare 350ml alkali solution.
[0049] 2. Salt solution preparation: weigh 72.1g Mg(NO 3 ) 2 ·6H 2 O, 52.8g Al(NO 3 ) 3 9H 2 O, 24.5gCe(NO 3 ) 3 ·6H 2 O and 36.6g La(NO 3 ) 3 ·6H 2 O crystals were put into a beaker, an appropriate amount of twice distilled water was added, and stirred to completely dissolve the crystals, and a 350ml salt solution was prepared.
[0050] 3. Hydrotalcite preparation: put the flask into a constant temperature water bath and fix it on an iron stand, and add 300ml of deionized water into the flask. Adjust the temperature of the constant temperature water bath to 65°C, and start the stirrer. The salt solution and the alkali solution are fed into the flask in parallel by adopting a double-drop feeding method, and the pH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com