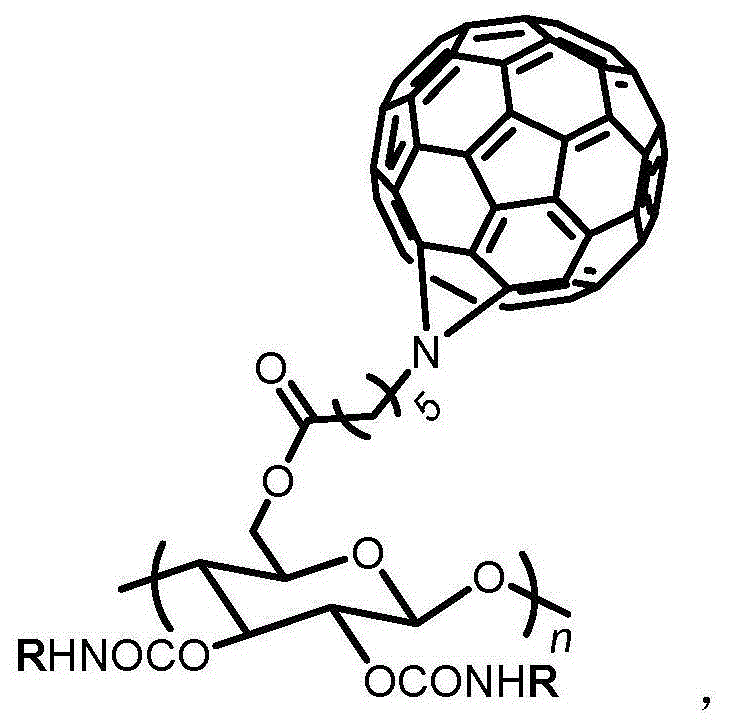
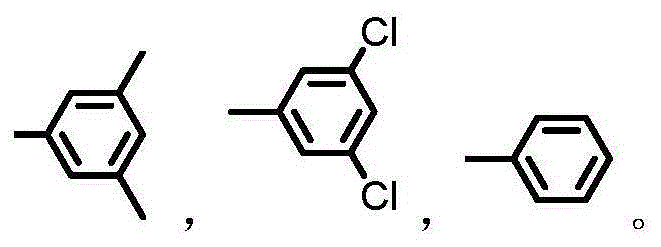
Fullerene cellulose derivative used as chiral stationary phase material and preparation method of fullerene cellulose derivative
A cellulose derivative, chiral stationary phase technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of limited types of chiral compounds to be separated, shortened service life of chiral stationary phase materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
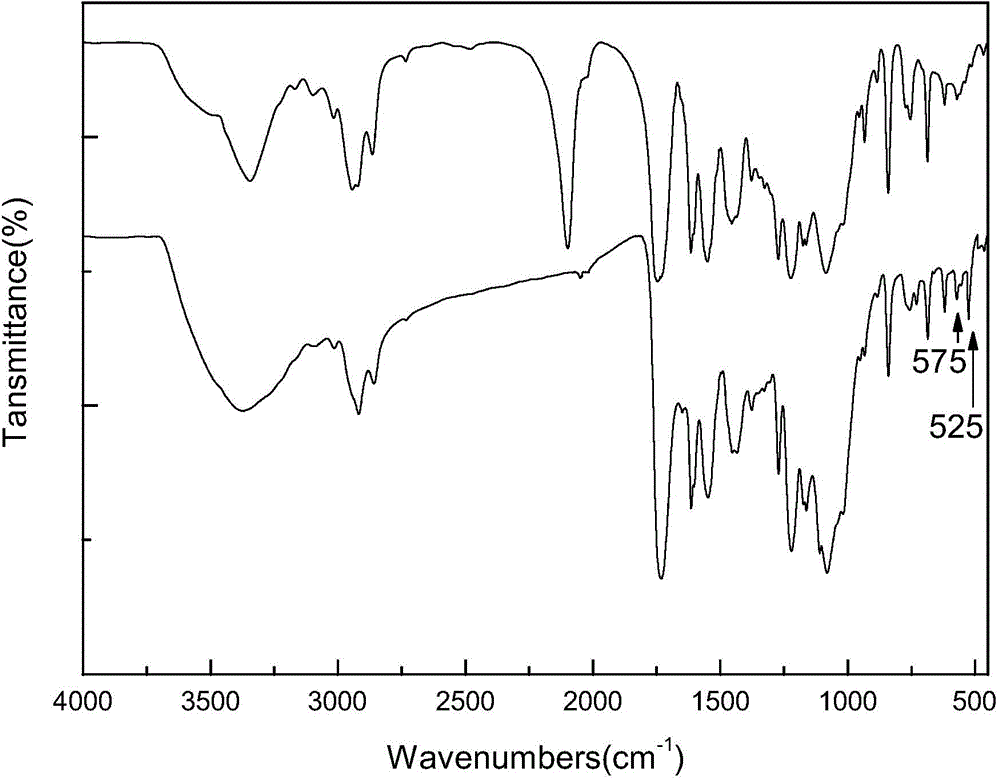
[0025] The whole process of the preparation method of the fullerene cellulose derivative used as the chiral stationary phase material of the present invention is as follows:
[0026]
[0027] The preparation process of the fullerene cellulose derivatives of the present invention will now be described in detail with examples.
[0028] 1. Preparation of cellulose-6-triphenylmethyl ether
[0029] Take 2 g of microcrystalline cellulose in a 250 mL two-necked bottle, and dry it in vacuum at 80° C. for 4 hours. Fill with dry high-purity nitrogen, add 60 mL of purified N,N-dimethylacetamide under nitrogen protection, and stir at 80°C for 12 hours. Cool to room temperature, add 4g of anhydrous lithium chloride as a catalyst, stir vigorously until the solution turns from cloudy to clear, then add 28mL of pyridine and 10.8g of triphenylchloromethane in sequence. Raise the temperature to 80°C and react for 24 hours. After the reaction, the orange-yellow product solution was added d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com