Immunity enhancing reagent
A technology of immune enhancement and reagents, applied in the field of biomedicine, can solve the problems of insurmountable immunosuppression and low curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
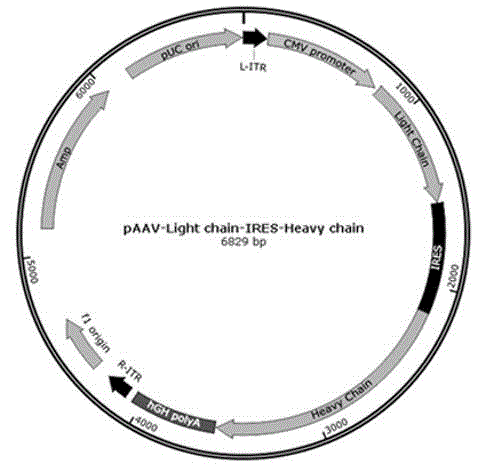
[0048] Example 1 Construction and preparation of immunoenhancing reagents
[0049] (1) According to the corresponding relationship in Table 1, synthesize 10 full-length DNA sequences that can encode the heavy chain and light chain of the immune checkpoint monoclonal antibody. The full-length heavy chain DNA sequence is composed of signal peptide DNA sequence, heavy chain variable region DNA sequence, heavy chain constant region DNA sequence; the light chain DNA full-length sequence is composed of signal peptide DNA sequence, light chain variable region DNA sequence, light chain The DNA sequence of the chain constant region is composed, and the specific sequence is shown in the sequence listing.
[0050]Table 1 The amino acid sequence and nucleotide base sequence corresponding to the variable region of the antibody molecule encoding the immune checkpoint and the code of the virus constructed in the present invention
[0051]
[0052] A total of 5 heavy chain full-length DNA...
Embodiment 2
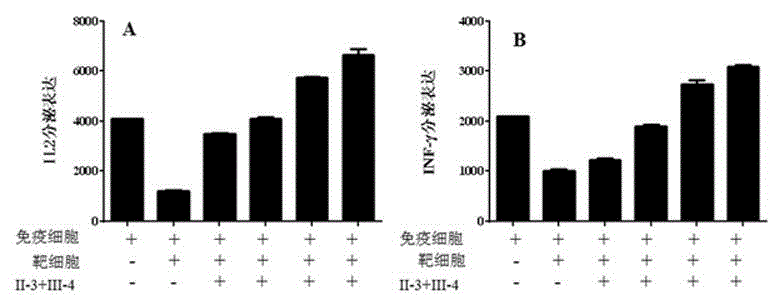
[0067] Example 2 Experimental verification of cytokine release in vitro
[0068] Peripheral blood mononuclear cells (PBMC cells) were isolated from human peripheral blood using Lymphoprep reagent, cultured with RPMI1640 and 10% FBS, and divided into blank group, control group, and experimental group.
[0069] The first group added PHA to the culture medium to activate peripheral blood T cells, which was the blank group; the second group was the co-culture group of PHA-activated T cells and tumor cells (lung cancer cells HCC829). Tumor cells were treated for 24 hours to increase the expression level of immune checkpoint molecular ligands (such as PD-L1 molecules, etc.) The cells were co-cultured and served as the control group; the experimental group was based on the second group of co-culture system, respectively adding different immunoenhancing reagents obtained from Example 1 (use normal saline to adjust the virus titer to the same concentration of 5× 10 13 vg / mL); 48 hou...
Embodiment 3
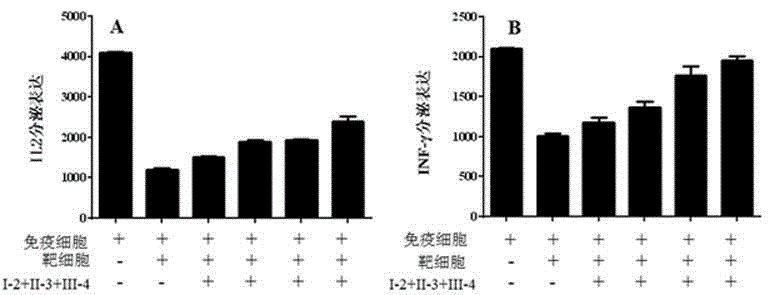
[0075] Example 3 Induction of apoptosis of tumor cells by immune cells secreting immune checkpoint antibodies
[0076] Peripheral blood mononuclear cell (PBMC) cells were isolated from human peripheral blood using Lymphoprep reagent, and cultured with RPMI1640 and 10% FBS. Add PHA to the medium to activate peripheral blood T cells as effector cells; treat tumor cells with γ-interferon for 24 hours to increase the expression level of immune checkpoint ligand molecules on the surface of tumor cells, such as PD-L1 molecules, on the tumor surface , and then washed three times with PBS to remove interferon-gamma in the medium, and tumor cells (hepatoma cells HepG2) treated with interferon-gamma were used as target cells. The activated effector cells were divided into two groups, and one of them was added to an immune enhancement reagent in Example 1 (immune checkpoint adeno-associated virus combination I-2:II-3:III-4=1:3:1 ), to infect the effector cells so that they can secrete a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com