Antimicrobial and anti-tumor emodin compound and application thereof
A technology of compounds and derivatives, which is applied in the application fields of antitumor, antibacterial and anti-inflammatory drugs, and can solve the problems of high toxicity of emodin, insufficient biological activity, and unclear mechanism of biological activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
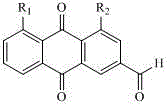
[0063] Embodiment one: the synthesis of ethyl rhein
[0064]Add 0.22 g of rhein into the reaction flask, add 100 ml of dichloromethane, add 5 ml of thionyl chloride under stirring, and react overnight under reflux until the system is dissolved. Evaporate the solvent under reduced pressure to dryness, add 5 ml of toluene to remove the excess toluene, and repeat 3 times to obtain rheinyl chloride.
[0065] 0.436 grams of rhein chloride was added to the reaction flask, 20 milliliters of dichloromethane was added, stirred, 2 milliliters of absolute ethanol and 0.5 milliliters of triethylamine were added at room temperature, and the reaction was carried out at room temperature. TLC followed the reaction (ethyl acetate:petroleum Ether = 1:2), after 32 hours, there was no significant change in the system, the solvent was evaporated under reduced pressure, separated by column chromatography, and 17 mg of yellow solid was obtained, with a yield of 7.7%.
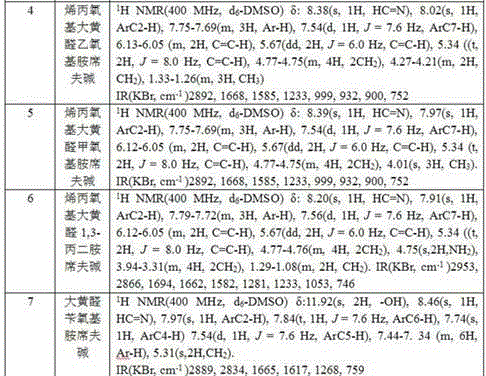
[0066] 1H NMR (400 MHz, d6-DM...
Embodiment 2
[0067] Embodiment two: the synthesis of methoxy pentyl rhein
[0068] Add 0.22 g of rhein into the reaction flask, add 100 ml of dichloromethane, add 5 ml of thionyl chloride under stirring, and react overnight under reflux until the system is dissolved. Evaporate the solvent under reduced pressure to dryness, add 5 ml of toluene to remove the excess toluene, and repeat 3 times to obtain rheinyl chloride.
[0069] Add 0.40 g of rhein to the reaction flask, add 20 ml of dichloromethane, stir, add 2 ml of n-amyl alcohol and 0.5 ml of triethylamine at room temperature, react at room temperature, follow the reaction by TLC (ethyl acetate:petroleum ether =1:2), after 32 hours, there was no significant change in the system, the solvent was evaporated under reduced pressure, separated by column chromatography, and 0.0525 g (0.148 mmol) of a yellow solid was obtained.
[0070] Add 0.0525 grams of n-pentyl rhein to the reaction flask, add 5 milliliters of dioxane, stir to dissolve, ad...
Embodiment 3
[0072] Embodiment three: the synthesis of rhein amide
[0073] Add 20 ml of anhydrous methanol to the reaction flask, add 2 ml of thionyl chloride dropwise under ice bath, after dropping, keep warm for 1 hour, add 1.3653 g of arginine, raise to room temperature, react for 4 hours, and heat up to 50°C , reacted for 16 hours, evaporated the arginine methyl ester of the solvent under reduced pressure.
[0074] Add 0.338 g of rhein to the reaction flask, add 0.2027 g of DCC, 0.1525 g of DMAP, 0.235 g of L-arginine methyl ester, and 20 ml of 1,2-dichloromethane, stir, heat up to 80°C, and keep warm for 23 hour, evaporate the solvent under reduced pressure, add 20 ml of anhydrous methanol to the residue, stir, filter off insoluble matter, evaporate most of the solvent from the filtrate under reduced pressure, filter with suction, and wash the filter cake with ether to obtain a yellow solid, which after drying Obtained 0.0738 grams of product, yield 13.7%.
[0075] 1H NMR (400 MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com