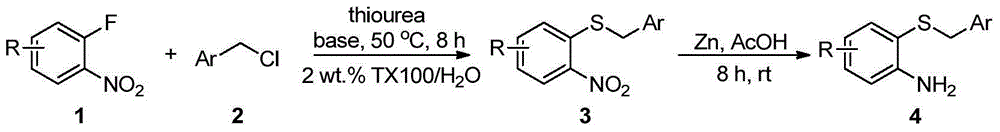Method for synthesizing poly-substituted 2-aryl benzothiazoles by utilizing thiourea as sulphur source
A benzothiazole and multi-substitution technology, which is applied in the field of synthesis of multi-substituted 2-aryl benzothiazoles, can solve the problems of limited range of reaction substrates, poor reaction selectivity, harsh reaction conditions, etc. Mild conditions and good substrate tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
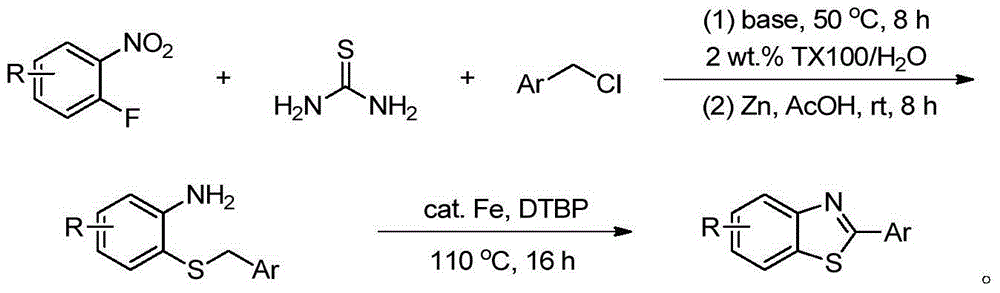
[0016] Embodiment 1: Preparation of o-aminophenyl benzyl sulfide 4
[0017] The reaction process of o-aminophenyl benzyl sulfide is as follows:
[0018]
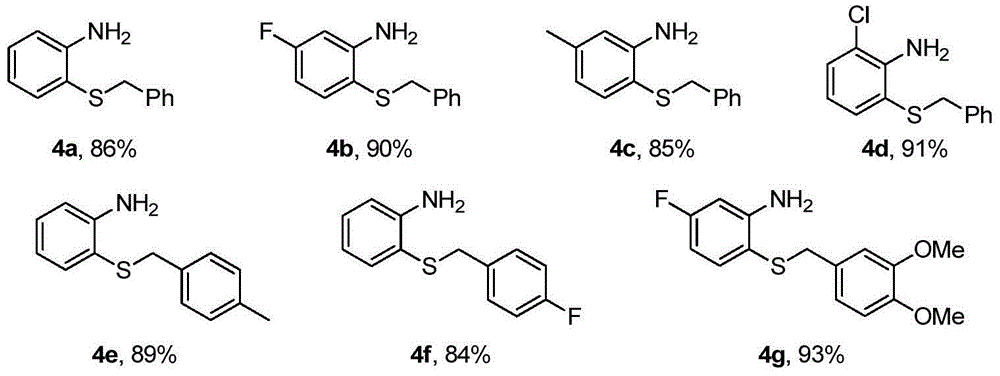
[0019] Add 1mmol o-fluoronitrobenzene 1, 1.5mmol thiourea, 1.2mmol benzyl chloride 2, 2mmol base and 5mL 2wt.%TX100 / H 2 O was sequentially added into a 25mL one-necked flask, and reacted at 50°C for 8h. After the reaction, it was cooled to room temperature, and 3 mmol of zinc powder and 4 mmol of acetic acid were directly added without post-treatment, and stirred at room temperature for 8 h. After the reaction, the solid powder was removed by suction filtration, extracted three times by adding ethyl acetate, the organic phase was collected and dried, and the organic phase was removed to obtain a crude product. Finally, pure o-aminophenylbenzyl sulfide 4a-4g was obtained through column chromatography on silica gel, producing The rates are shown below.
[0020]
Embodiment 2
[0021] Example 2: Preparation of multi-substituted 2-arylbenzothiazoles 5
[0022]
[0023] Add 1mmol o-aminophenyl benzyl sulfide 4, 10mol% iron catalyst, 3mmol di-tert-butyl peroxide and 5mL toluene into a 25mL single-necked flask in sequence, and react at 110°C for 16h. After the reaction, cool to room temperature, remove the solvent by rotary evaporation, and finally obtain the target product 5a-5g through column chromatography on silica gel, and the yield is as follows. Among them, GW610 (5g) is an antitumor drug.
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com