14C-labeled polystyrene (PS) and synthesis method thereof
A technology of polystyrene and styrene, applied in the field of synthesis of 14C-labeled polystyrene, which can solve the problem of low degree of polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
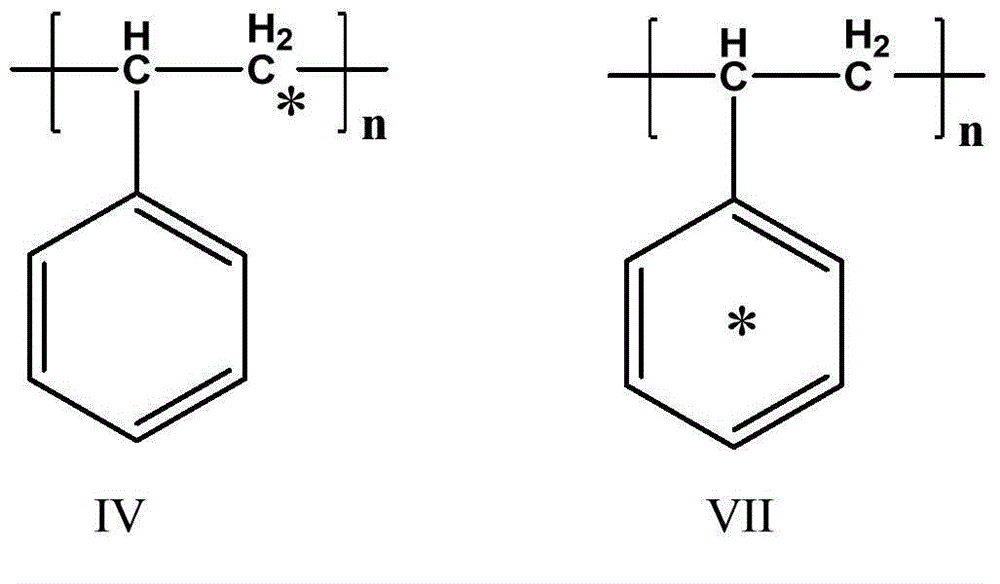
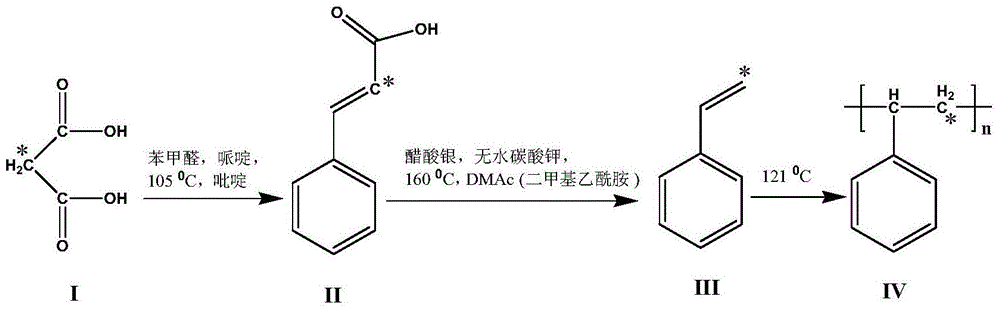
[0050] Example 1: branched chain 14 C-labeled [β- 14 C]-polystyrene ([β- 14 Synthesis of C]-PS)(IV)
[0051] combine figure 1 , 2, branch chain 14 C-labeled [β- 14 C]-polystyrene ([β- 14 C]-PS) (IV) synthetic steps are:
[0052] (1) Synthetic branched chain 14 C-labeled cinnamic acid ([β- 14 C]-cinnamic acid) (II):
[0053] Set the specific activity to 2.1×10 9 Bq mmol -1 of 14 C-labeled malonate 14 CH 2 (COOH) 2 (1) and appropriate unmarked malonic acid are dissolved in pyridine, join in the 25mL pear-shaped bottle, obtain specific activity and be 4.2 * 10 7 Bq mmol -1 of 14 C-malonic acid. Add 0.1128 g of benzaldehyde and 177.5 μL of piperidine in sequence, 14 CH 2 (COOH) 2 (1) The molar ratio with benzaldehyde is 1:1.2~1.5, nitrogen protection, magnetic stirring, oil bath 105 ℃, react for 14 hours. After the reaction, cool to room temperature, add 20 mL of 1M hydrochloric acid for acidification, and make the pH of the solution acidic. Extracted 9 time...
Embodiment 2
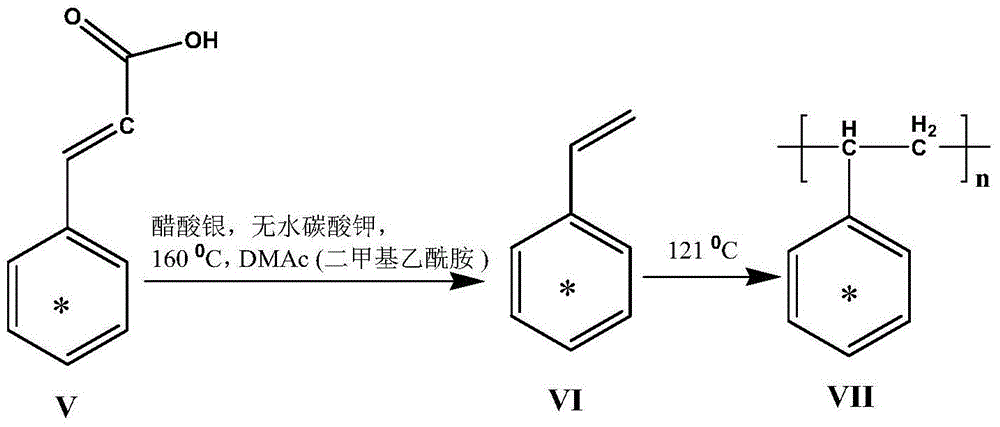
[0059] Example 2: benzene ring 14 C marked [U-ring- 14 C6]-polystyrene ([U-ring- 14 Synthesis of C6]-PS)(VII)
[0060] combine figure 1 , 2, benzene ring 14 C marked [U-ring- 14 C6]-polystyrene ([U-ring- 14 C6]-PS)
[0061] The synthetic steps of (VII) are:
[0062] (1) Synthesis of benzene ring 14 C labeled styrene ([U-ring- 14 C6]-styrene) (VI)
[0063] Take the specific activity as 1.3×10 8 Bq mmol -1 benzene ring 14 C-labeled cinnamic acid [U-ring- 14 C6]-cinnamic acid (V) and an appropriate amount of non-labeled cinnamic acid were added to a 25mL pear-shaped bottle, dissolved in 1mL DMAc (dimethylacetamide), and the specific activity was obtained: 1.3×10 7 Bq mmol -1 The [U-ring- 14 C6]-cinnamic acid. Add silver acetate 0.0029g, anhydrous potassium carbonate 0.0036g, [U-ring- 14 C6]-cinnamic acid, silver acetate and potassium carbonate in a molar ratio of 1:0.2 to 0.5:0.3 to 0.8, nitrogen protection, magnetic stirring, oil bath at 160°C, and reaction for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com