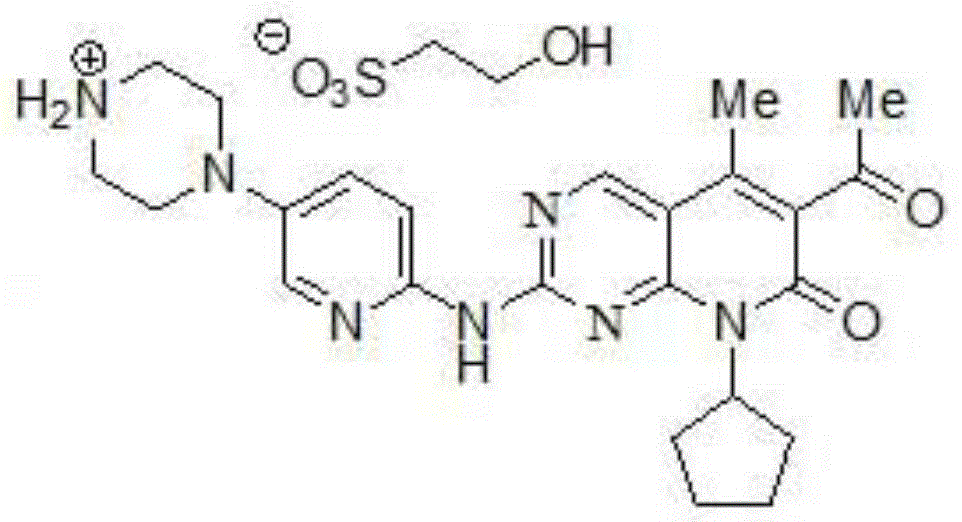
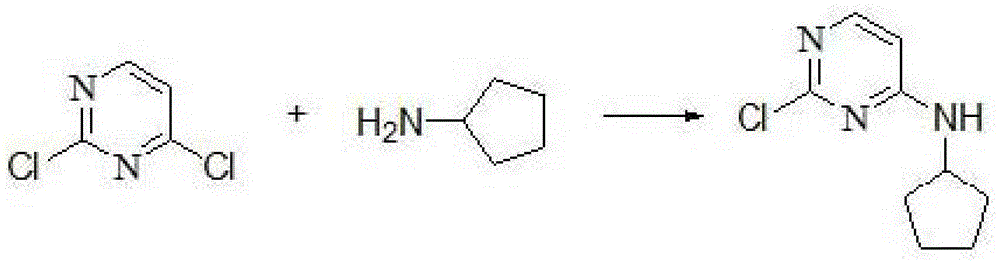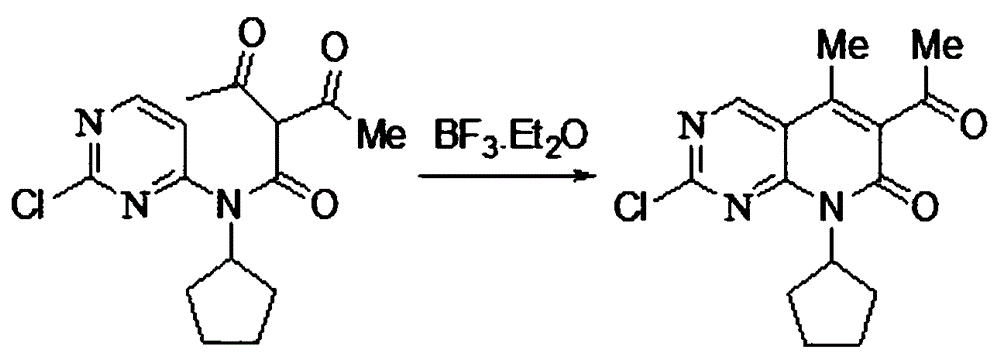Palbociclib preparation method
A refining method and solid technology, applied in the direction of organic chemistry, etc., can solve the problems of high cost, unfriendly environment, affecting process amplification, etc., and achieve the effect of low manufacturing cost and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A preparation method of Palbociclib, comprising the steps of:
[0027] (1) Substitution step: dissolving 2,4-dichloropyrimidine in an organic solvent, adding cyclopentylamine dropwise under stirring for reaction, and recrystallizing to obtain solid A;
[0028] (2) Dehydration step: dissolving the obtained solid A and 2,2-diacetylacetic acid in an organic solvent, adding dicyclohexylcarbodiimide dropwise under stirring conditions, and recrystallizing to obtain solid B;
[0029] (3) Cyclization step: dissolving the obtained solid B in an organic solvent, reacting under the catalysis of boron trifluoride ether, and refining to obtain solid C;
[0030] (4) Step of introducing side chains: the obtained solid C is dissolved in toluene as the first phase, and 4-(6-amino-pyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester is dissolved in toluene as the second phase Phase, the first phase is added dropwise during the stirring process of the second phase, after the react...
Embodiment 1
[0034] (1) Add 2 L of ethanol to a 5 L three-necked flask, then add 2,4-dichloropyrimidine (100 g, 0.67 mol), add cyclopentylamine (257 g, 3 mol) while stirring, and stir at room temperature for 3.5 hours. Add 4 L of water, continue stirring for 50 minutes, filter, collect the solid, and recrystallize with a 1:3 mixture of ethyl acetate and petroleum ether to obtain solid A (123 g, 89%). The main reaction equation of this step is:
[0035]
[0036] (2) Add 3L of dichloroethane to a 5L three-necked flask, add solid A (120g, 0.6mol) and 2,2-diacetylacetic acid (86g, 0.6mol) respectively, stir for 10 minutes and then add dicyclohexyl Carbodiimide (127g, 0.57mol), stirred at a constant temperature of 45°C for 4.5 hours, cooled to 10°C and continued stirring for 2 minutes, filtered. The filtrate was concentrated, 3L of water was added, and stirred for 1 hour. The solid was collected by filtration and recrystallized from a 1:2 mixture of ethyl acetate and petroleum ether to obt...
Embodiment 2
[0045] (1) Add 2 L of ethanol to a 5 L three-necked flask, then add 2,4-dichloropyrimidine (100 g, 0.67 mol), add cyclopentylamine (257 g, 3 mol) while stirring, and stir at room temperature for 5 hours. Add 4 L of water, continue stirring for 1 hour, filter, collect the solid, and recrystallize with a 1:4 mixture of ethyl acetate and petroleum ether to obtain solid A (119 g, 90%).
[0046] (2) Add 3L of dichloroethane into a 5L three-necked flask, add solid A (110g, 0.55mol) and 2,2-diacetylacetic acid (79g, 0.55mol) respectively, stir for 10 minutes and then add dicyclohexyl Carbodiimide (117 g, 0.57 mol) was stirred at a constant temperature of 50° C. for 4 hours, cooled to 10° C. and stirred for 3 minutes, and filtered. The filtrate was concentrated, 3L of water was added, and stirred for 1 hour. The solid was collected by filtration and recrystallized from a 1:1 mixture of ethyl acetate and petroleum ether to obtain solid B (155 g, 87%).
[0047] (3) Add 2L of toluene to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com