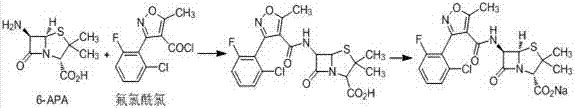
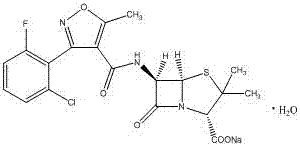
A kind of synthetic method of flucloxacillin sodium monohydrate
A technology of flucloxacillin sodium and monohydrate, which is applied in the field of drug synthesis, can solve the problems of large amount of organic solvents, high cost, and high pollution, and achieve the effects of increased yield, low cost, and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of flucloxacillin sodium monohydrate
[0033] Add 100 g of water into the reaction flask, add 20.0 g of 6-APA under stirring, and cool down to 0-5 °C. Weigh 10.6 g of sodium carbonate and dissolve it in 100 mL of water to make a 1 mol / L sodium carbonate solution; control the temperature below 10°C, and add the sodium carbonate solution dropwise into the reaction bottle to obtain a 6-APA sodium salt solution;
[0034] 28.0 g of 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carbonyl chloride was added into the reaction flask in 4 batches, and the addition time was controlled to be 1 hour. Keep warm at 20-25 ℃ for 4 hours; add 1 mol / L dilute hydrochloric acid dropwise to the above reaction solution, and adjust the pH to 2.5;
[0035] 400 g of ethyl acetate was added to the reaction flask, stirred, separated, and the ethyl acetate phase was separated. The ethyl acetate phase was washed with 200 g of saturated brine, separated, and then dried with 2...
Embodiment 2
[0038] Example 2 Preparation of flucloxacillin sodium monohydrate
[0039] Add 100 g of water into the reaction flask, add 20.0 g of 6-APA while stirring, and cool down to 0-5°C. Weigh 12.8 g of sodium carbonate and dissolve it in 80 mL of water to make a 1.5 mol / L sodium carbonate solution; control the temperature below 10°C, add the sodium carbonate solution dropwise into the reaction flask to obtain a 6-APA sodium salt solution;
[0040]Add 30.0 g of 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-formyl chloride into the reaction flask in 4 batches, and the feeding time is controlled at 1.5 hours; keep warm at 20-25 °C React for 3 hours; add 1 mol / L dilute hydrochloric acid dropwise to the above reaction solution, and adjust the pH to 3.0;
[0041] Add 300 g of methyl tert-butyl ether into the reaction flask, stir, separate liquids, and separate the organic phase. The organic phase was washed with 200 g of saturated brine, separated, and then dried with 20 g of anhydrous...
Embodiment 3
[0043] Example 3 Preparation of flucloxacillin sodium monohydrate
[0044] Add 100 g of water to the reaction flask, add 20.0 g of 6-APA while stirring, and cool down to 15-20 °C; weigh 13.8 g of potassium bicarbonate and dissolve it in 150 mL of water to prepare a 0.93 mol / L potassium bicarbonate solution. Control the temperature below 10°C, add potassium bicarbonate solution dropwise into the reaction flask to obtain 6-APA sodium salt solution;
[0045] Add 27.0 g of 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-formyl chloride into the reaction flask in 4 batches, and the feeding time is controlled at 1 hour; keep warm at 15-20°C React for 4 hours; add 2 mol / L dilute sulfuric acid dropwise to the above reaction solution, and adjust the pH to 3.0;
[0046] 200 g of dichloromethane was added to the reaction flask, stirred, separated, and the ethyl acetate phase was separated. The dichloromethane phase was washed with 200 g of saturated brine, separated, and then dried wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com