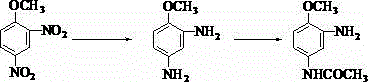Method for preparing 2-amino-4-acetamido anisole
A technology of acetamidoanisole and dinitroanisole, which is applied in the field of preparation of 2-amino-4-acetamidoanisole, can solve the problem of high equipment safety requirements, serious environmental pollution and harsh operating conditions To achieve the effect of safe and easy to control the reaction process, low equipment requirements, and increase product content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 100kg of 2,4-dinitroanisole, 50kg of hydrazine hydrate with a mass percentage of 80%, 10kg of iron acetylacetonate, and 500kg of methanol into a reaction flask with a condensing reflux device, and control the temperature at 65°C for 4 hours. , filtered and recovered the magnetic Fe 3 o 4 After powdering, 2,4-diaminoanisole-methanol mixture is obtained; then the obtained 2,4-diaminoanisole-methanol mixture is placed in a reaction bottle, and the ice bath (0 ° C ~ 5 ° C) conditions Add 75 kg of acetic anhydride dropwise while stirring, and control the dropwise addition within 40 minutes, and continue to keep the ice bath for 30 minutes under stirring, and recover methanol by distillation under reduced pressure, then filter and dry to obtain 2-amino-4-acetamidobenzene 89.5kg product of methyl ether (content: 99.7%, yield: 98.5%).
Embodiment 2
[0019] Add 350kg of 2,4-dinitroanisole, 250kg of hydrazine hydrate with a mass percentage of 80%, 52.5kg of nickel acetylacetonate, and 1750kg of tetrahydrofuran into a reaction flask with a condensing reflux device, and control the temperature to react at 70°C After 3 hours of filtration, the 2,4-diaminoanisole-tetrahydrofuran mixture was obtained; then the obtained 2,4-diaminoanisole-tetrahydrofuran mixture was placed in a reaction flask, and placed in an ice bath (0°C~5°C 280kg of acetic anhydride was added dropwise while stirring under the condition of stirring, and the dropwise addition was completed within 1 hour, and the ice bath was kept for 40 minutes under stirring, and tetrahydrofuran was recovered by distillation under reduced pressure, and then filtered and dried to obtain 2-amino-4-acetyl Anisoline 312kg product (content: 99.5%, yield: 98%).
Embodiment 3
[0021] Add 200kg of 2,4-dinitroanisole, 140kg of hydrazine hydrate with a mass percentage of 80%, 24kg of ruthenium acetylacetonate, and 700kg of ethanol in sequence in a reaction flask with a reflux device, and control the temperature at 70°C for 4 hours. After filtration, the 2,4-diaminoanisole-ethanol mixture was obtained; then the obtained 2,4-diaminoanisole-ethanol mixture was placed in a reaction flask, and placed in an ice bath (0°C~5°C) Add 180kg of acetic anhydride dropwise while stirring under the conditions, control the dropwise addition within 50min, and keep the ice bath condition under stirring for 40min, recover ethanol by distillation under reduced pressure, then obtain 2-amino-4-acetyl after filtration and drying Anisoline 178.5kg product (content: 99.2%, yield: 98.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com