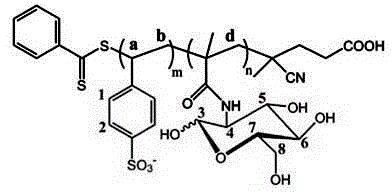
Novel glycosaminoglycan analogue and synthetic method thereof, and application of novel glycosaminoglycan analogue in invitro embryonic stem cell proliferation and directional neural differentiation
A technology for glycosaminoglycan and analogs, which is applied to novel glycosaminoglycan analogs and their synthesis and their application in in vitro embryonic stem cell proliferation and directed neural differentiation, and can solve the problems of limited yield and not widely obtained. application, cumbersome synthesis and purification steps, etc., to achieve the effects of high biological activity, easy process control, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A kind of synthetic method of novel glycosaminoglycan analogue, comprises the following steps:
[0034] (1) Select the molecule SS containing sulfonic acid groups and the molecule MAG containing sugar groups as monomers, and by adjusting the relative ratio of the two, use the RAFT method to prepare homopolymers and copolymers with different functional groups (molecular weight in 6000-20000 range).
[0035] (2) The obtained polymers were added to the embryonic stem cell culture medium to culture the embryonic stem cells, and after a certain period of time, the proliferation of cells in different polymer environments was studied.
[0036] (3) The obtained polymers were added to the embryonic stem cell culture medium respectively, and the embryonic stem cells were cultured, and after a certain period of time, the directed neural differentiation of the cells in different polymer environments was studied.
[0037] The principle of the present invention is: adopt the method ...
Embodiment 1
[0042] Preparation of monomer 2-methacrylamide glucopyranose (MAG): Glucosamine hydrochloride and methacryloyl chloride were reacted in a methanol solution containing potassium carbonate under ice bath conditions for 3 to 4 hours, then suction filtered and rotary evaporated to obtain a precipitate , and the precipitate was purified and dried to obtain the monomer MAG.
[0043] SS and MAG were selected as monomers, RAFT polymerization was carried out in H2O / DMF (1 / 1) mixed solvent, and the initial feeding ratio of monomers was adjusted to obtain homopolymer pSS, pMAG and copolymer pSG, respectively.
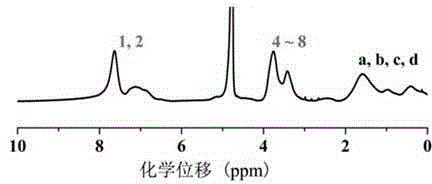
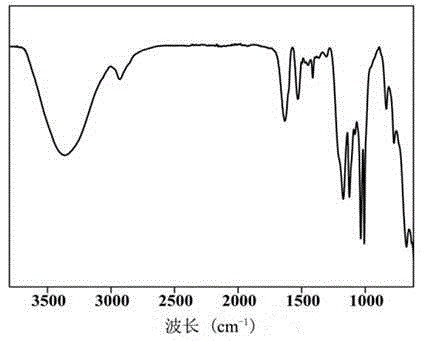
[0044] The obtained polymer was characterized by NMR and IR.
[0045] The result is as Figure 1-3 Shown, the 1H-NMR spectrum and FT-IR spectrum of polymer pSG.
[0046] The polymer molecule is used to promote the proliferation of embryonic stem cells in vitro, comprising the following steps:
[0047] The embryonic stem cells were seeded on a culture plate covered with ...
Embodiment 2
[0050] The embryonic stem cells were seeded on a culture plate pre-spread with gelatin, and cultured in a stem cell culture medium environment containing 1 μg / mL of the three polymers or heparin respectively for 10 days and 20 days.
[0051] Discard the solution in the culture, treat the embryonic stem cells with the cell culture solution containing 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT) for 4 hours, and suck Remove the MTT cell culture medium and add dimethyl sulfoxide (DMSO) to dissolve the formazan crystals generated in living cells.
[0052] The DMSO solution in which formazan crystals were dissolved was transferred to a new culture plate, and the absorbance value of each well was read at 490 nm on a microplate reader.
[0053] The result is as Figure 4 Shown are the proliferative behaviors of embryonic stem cells cultured in the medium containing the obtained polymer for 10 days and 20 days respectively.
[0054] The polymer molecule is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com