Bay-position oxygen-intercalation aza-heptatomic ring 3, 4:9, 10-perylene tetracarboxylic acid butyl acetate and synthesis method thereof
A technology of perylene tetracarboxylic acid and synthesis method, which is applied in the direction of organic chemistry, can solve the problems such as the difficulty in the synthesis of perylene compounds with seven-membered rings, and achieve the effects of high yield, mild reaction conditions, and good photophysical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
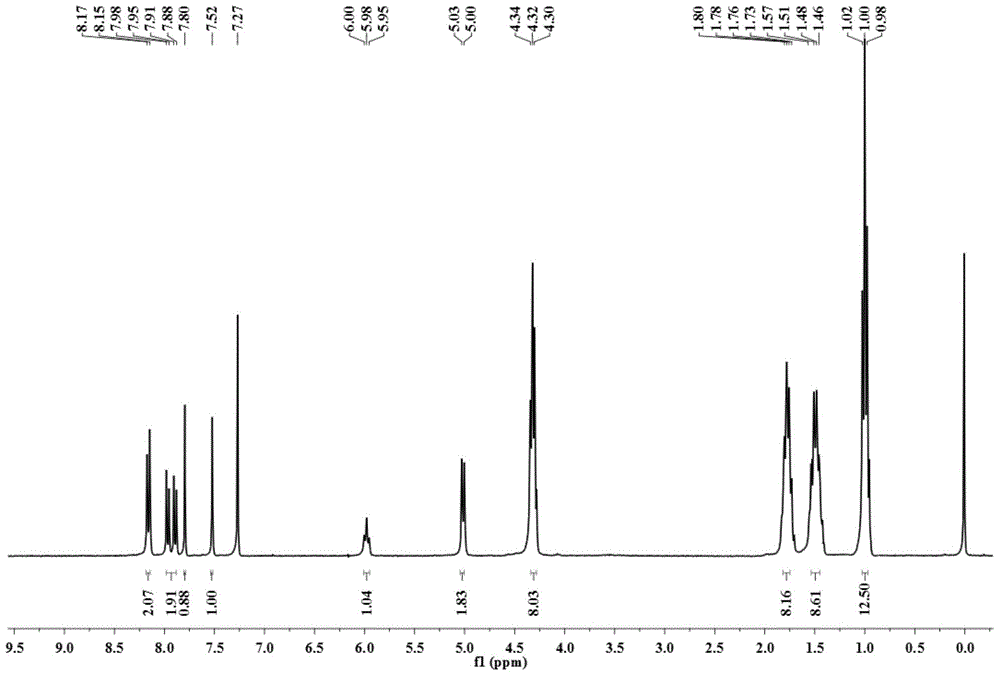
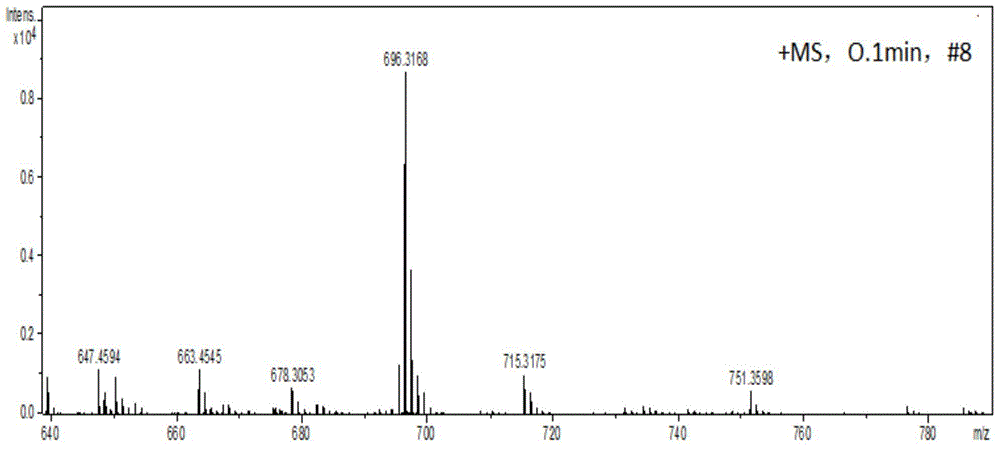
[0037] In a 100 ml round bottom flask, add 1-amino-12-hydroxyl-3,4:9,10-perylenetetracarboxylic acid n-butyl ester (200 mg, 0.29 mmol), paraformaldehyde (60 mg, 2.0 mg mol), dehydrated ethanol (50 milliliters), reacted for 12 hours at 78 degrees Celsius. After the reaction was completed, it was cooled to room temperature, and the solvent was removed to obtain a crude product. The crude product was purified by column chromatography (silica gel 200-300 mesh, eluent dichloromethane: ethyl acetate = 20:1 (volume ratio)) to obtain 123 mg of a red solid, which is compound A (structurally characterized as figure 1 with 2 ), yield 61%. 1 H-NMR (300MHz, CDCl 3 ,ppm):δ=8.16(d,J=6.0Hz,2H),7.96(d,J=9Hz,1H),7.89(d,J=9Hz,1H),7.80(s,1H),7.52(s ,1H),5.98(t,J=7.3Hz,1H),5.02(d,J=7.3Hz,2H),4.34-4.28(m,8H),1.80-1.73(m,8H),1.53–1.46( m,8H),1.02-0.98(m,12H).MS(MALDI-TOF):m / z=696.3168(M + ).
[0038] The paraformaldehyde was purchased from Sinopharm Chemical Reagent Co., Ltd., product number ...
Embodiment 2
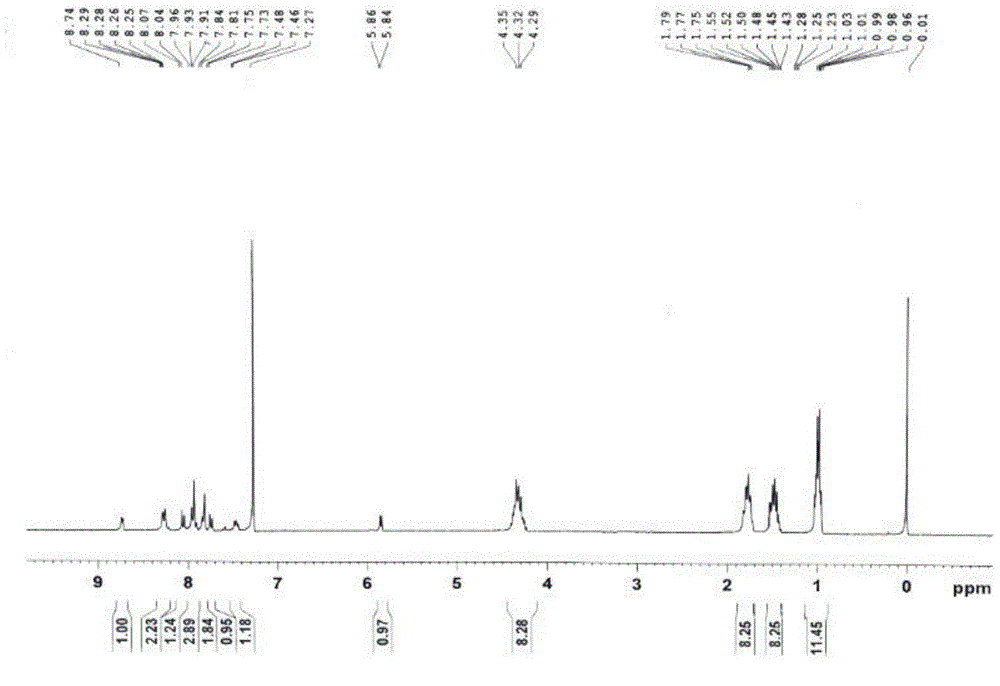
[0046] In a 50 ml round bottom flask, add 1-amino-12-hydroxyl-3,4:9,10-perylenetetracarboxylic acid n-butyl ester (200 mg, 0.29 mmol), pyridine-2-carbaldehyde (107 mg, 1 mmol), tetrahydrofuran (20 ml), and reacted at 65 degrees Celsius for 48 hours. After the reaction was completed, it was cooled to room temperature, and the solvent was removed to obtain a crude product. The crude product was purified by column chromatography (silica gel 200-300 mesh, eluent dichloromethane: ethyl acetate = 20:1 (volume ratio)), and 117 mg of a red solid was obtained, which was compound B (structural characterization as image 3 with 4 ), yield 52%.1 H-NMR (300MHz, CDCl 3 ,ppm): δ=8.73(d,J=6.0Hz,1H),8,28(d,J=3Hz,1H),8.25(d,J=3Hz,1H),8.05(d,J=7.8Hz ,1H),7.96-7.93(m,3H),7.84-7.81(m,2H),7.74(d,J=7.8Hz,1H),4.35-4.29(m,8H),1.79-1.75(m,8H ),1.52–1.43(m,8H),1.03-0.96(m,12H).MS(MALDI-TOF):m / z=773.3508(M + ).
[0047] The source of the 1-amino-12-hydroxyl-3,4:9,10-perylenetetracarboxylic acid n-b...
Embodiment 3
[0054] In a 50 ml round bottom flask, add 1-amino-12-hydroxyl-3,4:9,10-perylenetetracarboxylic acid n-butyl ester (200 mg, 0.29 mmol), 8-hydroxyquinoline-2-formaldehyde (173 mg, 1 mmol), 1,4-dioxane (30 ml), reacted at 80°C for 10 hours. After the reaction was completed, it was cooled to room temperature, and the solvent was removed to obtain a crude product. The crude product was purified by column chromatography (silica gel 200-300 mesh, eluent dichloromethane: ethyl acetate = 20:1 (volume ratio)), and 103 mg of red solid was obtained, which was compound C (structural characterization as Figure 5 with 6 ), yield 55%. 1 H-NMR (300MHz, CDCl 3 , ppm): δ=8.36(d, J=9.0Hz, 1H), 8.30(s, 1H), 8.10-8.05(m, 2H), 7.97(d, J=9Hz, 1H), 7.84-7.81(m ,2H),7.75(d,J=9Hz,1H),7.62-7.56(m,2H),7.46(d,J=9Hz,1H),7.34(d,J=6Hz,1H),5.76(d, J=6Hz, 1H), 5.02(d, J=7.3Hz, 2H), 4.44-4.31(m, 8H), 1.89-1.77(m, 8H), 1.53–1.40(m, 8H), 1.07-1.02( m,12H).MS (MALDI-TOF): m / z=839.3389 (M + ).
[0055] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com