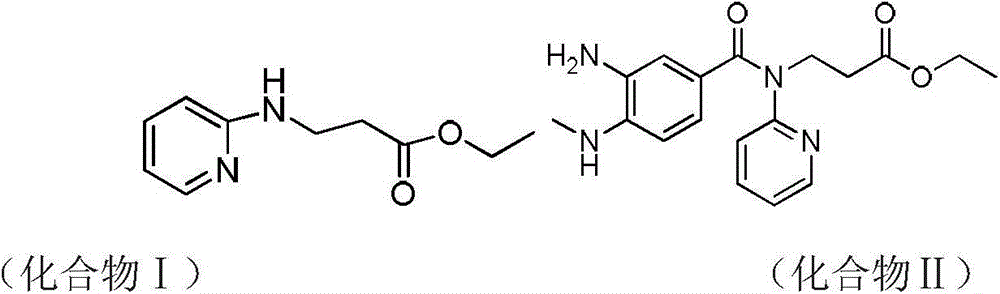
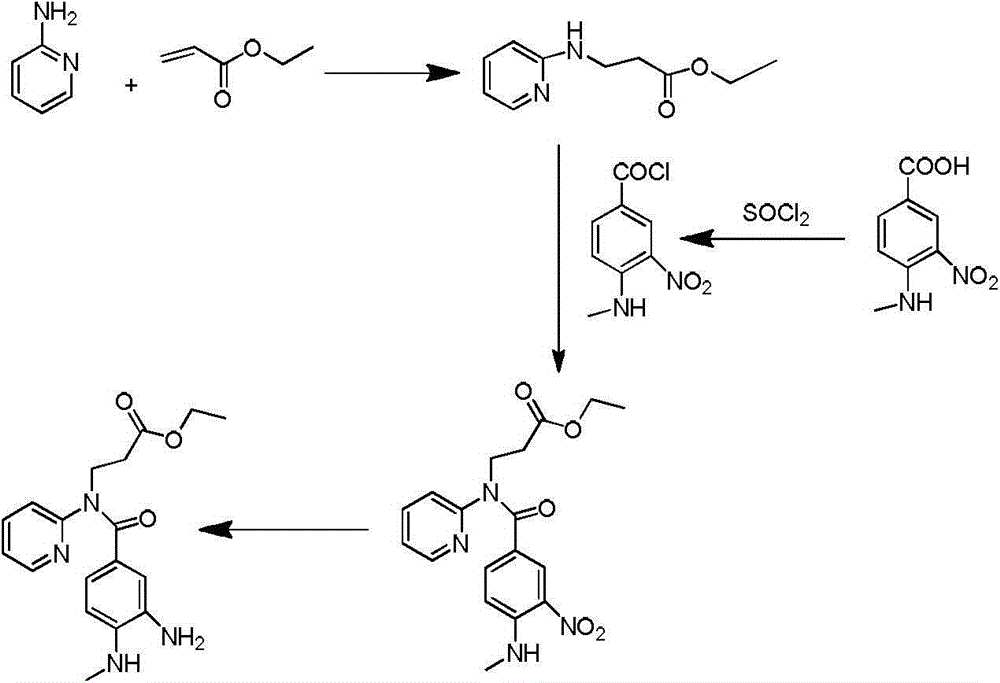
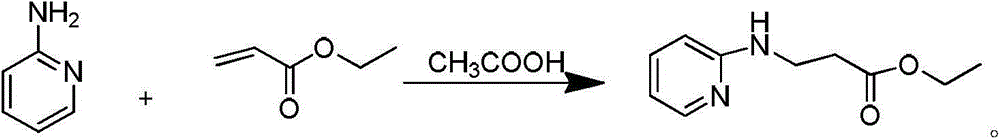Synthetic method of 3-(pyridine-2-yl-amino) ethyl propionate
A synthetic method, the technology of ethyl propionate, applied in the direction of organic chemistry, etc., can solve problems such as unsuitable for industrial production, unsuitable for industrial production, explosion of ethyl acrylate, etc., achieve low equipment performance requirements, improve production safety, The effect of short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: synthetic 3-(pyridin-2-ylamino) ethyl propionate
[0030] Add 20.0g of 2-aminopyridine, 276.8ml of ethyl acrylate and 6.36ml of glacial acetic acid to a 100ml three-necked flask in sequence, raise the external temperature to 85°C, and the internal temperature to 80°C, stir overnight, and monitor the reaction by hplc. After 12h, 2-amino Pyridine reaction is complete, lower the system to room temperature, then add 110ml 2N HCl and stir, there is some exotherm during the stirring process, add 50ml ethyl acetate for extraction after 30min, separate liquids, collect the water phase, and then use 50ml ethyl acetate to dilute the water phase Washing, liquid separation, combined water phase, then add 15.8g sodium carbonate solid and 100ml ethyl acetate to extract under stirring, separate liquid to collect organic phase, then use 25ml ethyl acetate to extract water phase, combine organic phase, spin dry ethyl acetate. Then 34g DMF and 170g water were added, stirre...
Embodiment 2
[0031] Embodiment 2: Synthetic 3-(pyridin-2-ylamino) ethyl propionate
[0032] Add 200g of 2-aminopyridine, 276.8ml of ethyl acrylate and 63.6ml of glacial acetic acid to a 1L three-necked flask in sequence, raise the external temperature to 85°C, and the internal temperature to 80°C, stir overnight, and monitor the reaction by hplc. After 12 hours, 2-aminopyridine After the reaction is complete, cool the system down to room temperature, then add 1100ml of 2N HCl and stir. There is some heat generation during the stirring process. After 30 minutes, add 500ml of ethyl acetate for extraction, separate the liquids, collect the water phase, and then wash the water phase with 500ml of ethyl acetate. , liquid separation, combined water phase, then add 158g sodium carbonate solid and 1L ethyl acetate to extract under stirring, separate liquid to collect organic phase, then use 250ml ethyl acetate to extract water phase, combine organic phase, spin dry acetic acid ethyl ester. Then, ...
Embodiment 3
[0033] Embodiment 3: Synthetic 3-(pyridin-2-ylamino) ethyl propionate
[0034]Add 130kg of 2-aminopyridine, 174kg of ethyl acrylate and 44kg of glacial acetic acid to a 2000L three-necked flask in sequence. The external temperature rises to 85°C, and the internal temperature rises to 80°C. Stir overnight, and hplc monitors the reaction. After 12 hours, the reaction of 2-aminopyridine is complete , the system was lowered to room temperature, then added 72L2N HCl and stirred, there was some exotherm during the stirring process, 300min later, 300kg ethyl acetate was added for extraction, the liquid was separated, the water phase was collected, and then the water phase was washed with 300kg ethyl acetate and separated liquid, combined the aqueous phase, and then added 103kg of sodium carbonate solid and 300kg of ethyl acetate under stirring for extraction, separated and collected the organic phase, then extracted the aqueous phase with 150kg of ethyl acetate, combined the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com