Sodium prasterone sulfate dispersible tablet and preparation method thereof
A technology of prasterone sulfate sodium and dispersible tablets, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulas, etc., can solve problems that need to be deepened, and achieve good uniformity and preparation methods. Simple, good-tasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the prescription screening of Prasterone Sulfate Sodium Dispersible Tablets
[0039] Take the prasterone sulfate sodium dispersible tablet of 100 mg as an example.
[0040] The preparation method comprises the following steps:
[0041] 1) mixing prasterone sulfate sodium and a solubilizer, and micronizing it to particles with a particle size of 0.5-50 microns (μm), for subsequent use;
[0042] 2) Utilize a blender to uniformly mix the filler microcrystalline cellulose, the disintegrating agent crospovidone, the flavoring agent and magnesium stearate with the granules obtained in the above step 1;
[0043] 3) Utilize a high-speed tablet press to compress the powder obtained in the above step 2 to obtain prasterone sulfate sodium dispersible tablets.
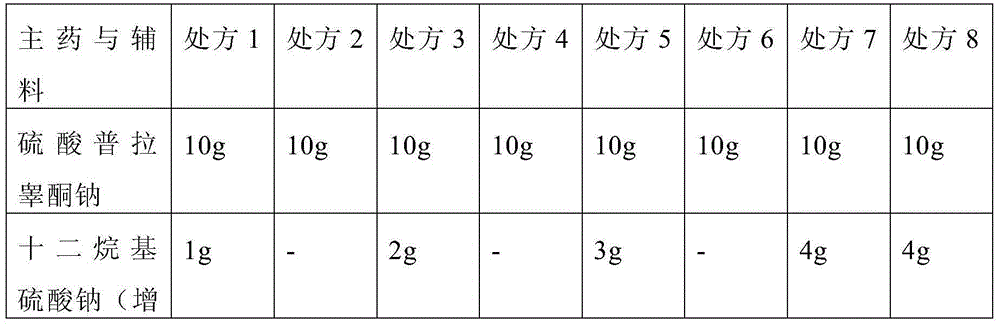
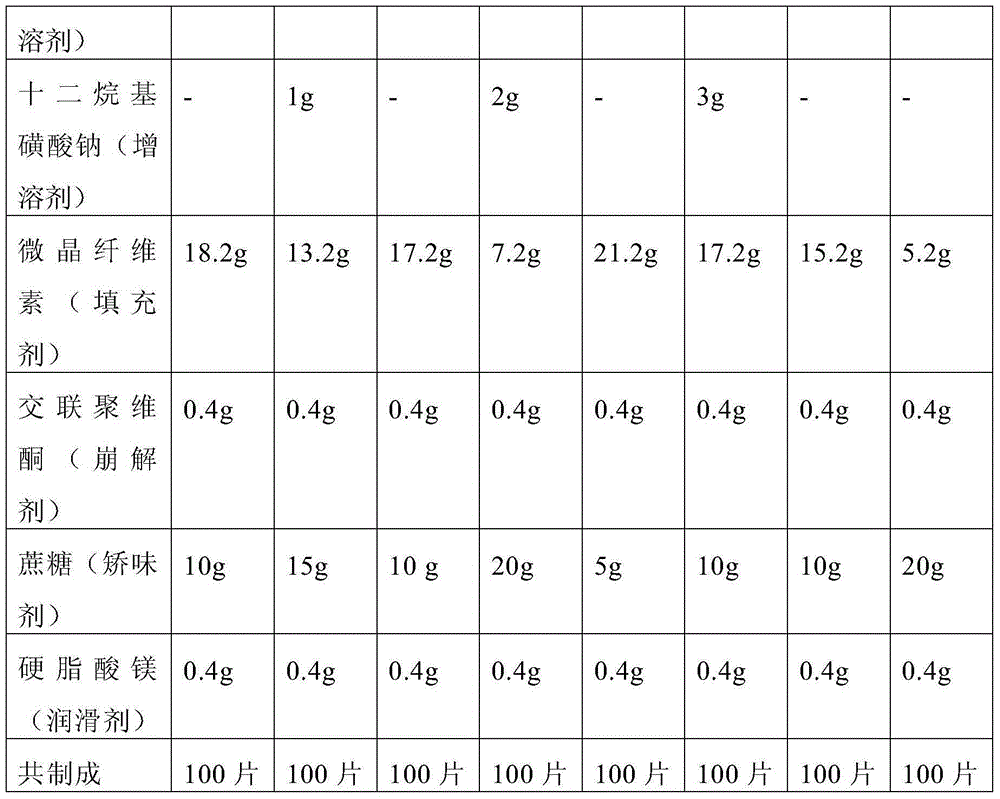
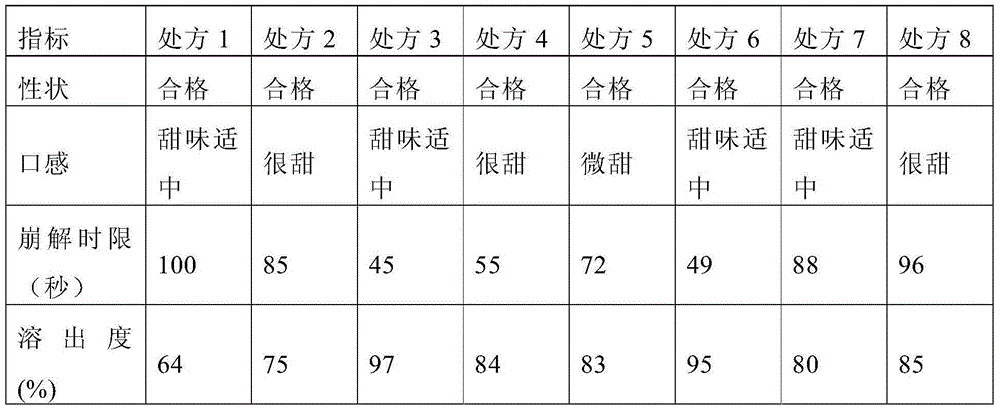
[0044] The prescription screening is shown in Table 1, and the prescription screening results are shown in Table 2.
[0045] Table 1 Prescription Screening Form
[0046]
[0047]
[0048] Table 2 Pre...
Embodiment 2
[0052] Prescription: prasterone sulfate sodium 10g, sodium lauryl sulfate 2g, microcrystalline cellulose 17.2g, crospovidone 0.4g, sucrose 10g, magnesium stearate 0.4g, made into 100 tablets.
[0053] Preparation Process:
[0054] 1) Mixing prasterone sodium sulfate and sodium lauryl sulfate in the prescribed amount, micronized to particles with a particle size of 0.5-50 microns (μm), and set aside;
[0055] 2) Utilize the mixing machine to mix the microcrystalline cellulose, crospovidone, sucrose and magnesium stearate in the prescribed amount with the above-mentioned step 1;
[0056] 3) Utilize a high-speed tablet press to compress the powder in the above step 2 to obtain prasterone sulfate sodium dispersible tablets.
[0057] In addition, the prasterone sulfate sodium dispersible tablet preparation prepared by this prescription has been carried out 30 days influence factor experiment, to investigate the stability of pharmaceutical preparation, the stability data experiment...
Embodiment 3
[0063] Prescription: prasterone sulfate sodium 10g, sodium lauryl sulfonate 2g, microcrystalline cellulose 20g, croscarmellose sodium 0.4g, sucrose 10g, magnesium stearate 0.4g, made into 100 tablets.
[0064] Preparation process: with embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com