Marine fungus-derived polyketone compound and application thereof in treatment of type 2 diabetes
A technology of polyketides and marine fungi, applied in the fields of microorganisms, metabolic diseases, organic chemistry, etc., can solve the problems of lack of specific drugs, etc., achieve significant inhibitory activity, simple method, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The compounds of the present invention can be obtained from marine fungi Aspergillus isolated from the fermentation broth of sp. 16-5B. marine fungi Aspergillus sp. 16-5B is a mangrove plant from Dongzhaigang Mangrove National Nature Reserve, Hainan Island Sonneratia apetala isolated from leaves. Marine fungus Aspergillus Aspergillus The sp. 16-5B strain was deposited in the China Center for Type Culture Collection on April 6, 2015 at Wuhan University, Wuhan, China, with the accession number CCTCC M 2015204.
[0028] The separation method of polyketide is as follows:
[0029] S1. Seed culture:
[0030] S11. Prepare seed medium: glucose 40g, peptone 4g, yeast extract 4g, sea salt 5g, tap water 2000mL, evenly distribute in 8 500mL Erlenmeyer flasks, extinguish at 121°C for 25 minutes.
[0031] Cultivation of seeds: the marine fungus Aspergillus The sp. 16-5B strain was inoculated into the seed medium, and placed on a shaker at a speed of 150 rpm at a tempe...
Embodiment 2
[0039] The compound in embodiment 1 is carried out structural analysis test, obtains following physical and chemical property data:
[0040] White powder, melting point 121.1-121.6 °C (thermometer uncalibrated), EI-MS (m / z): 372 [M] + ; HR-EI-MS (m / z): 372.1209 [M] + (Theoretical value 372.1204).
[0041] 1 H NMR (500 MHz, CDCl 3 ) δ 11.27 (s, 1H), 6.58 (dd, J = 15.8, 6.7 Hz, 1H), 6.28 (dd,J = 15.8, 1.4 Hz, 1H), 6.16 (s, 1H), 6.08 (d, J = 1.8 Hz, 1H), 6.03 (d, J = 1.8 Hz, 1H), 5.11 (dd, J = 10.5, 6.1 Hz, 1H), 3.07 (dd, J = 16.6, 5.5 Hz, 1H), 2.70 (dd, J = 16.6, 10.5 Hz, 1H), 2.39 (s, 3H), 1.83 (d, J = 6.7 Hz, 3H), 1.56 (s, 3H).
[0042] 13 C NMR (125 MHz, CDCl 3 ) δ 181.9, 170.3, 164.9, 161.6, 161.0, 144.2, 144.1, 138.7, 134.2, 119.0, 112.2, 107.8, 104.8, 101.2, 87.3, 69.6, 24.4, 18.9, 16.
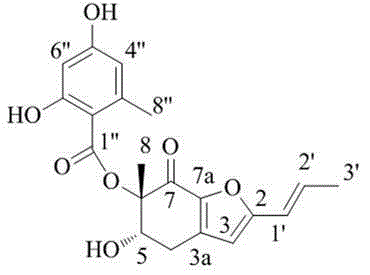
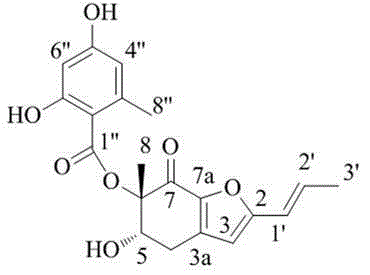
[0043] According to the above data, it is known that the structural formula of polyketides is as shown in formula (I):
[0044]
[0045] Formula (Ⅰ).
Embodiment 3
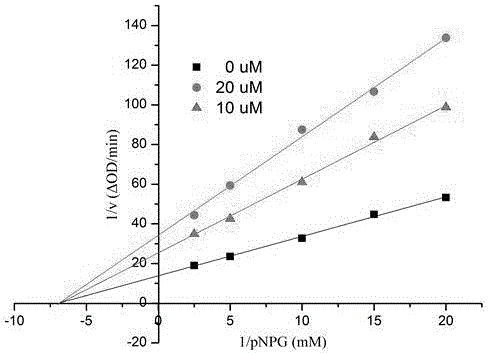
[0047] The compound in embodiment 1 is carried out α-glucosidase inhibition experiment:
[0048] Using p-nitrophenol-α-glucoside (pNPG) as the substrate, carried out in 0.01M phosphate buffer (pH7.0). pNPG was enzymatically hydrolyzed into p-nitrophenol by α-glucosidase, and the enzyme activity was calculated by measuring the change of its absorbance at a wavelength of 400nm with a UV-visible spectrophotometer. Sample and positive control (acarbose) were all made into DMSO solution (both 10 mu mol / mL), the enzyme and the substrate were formulated with 0.01M phosphate buffer solution at a suitable concentration, 1mL initial reaction system contained 0.1unit enzyme, 60 mu L substrate, 20 mu L DMSO. Take an appropriate amount of enzyme solution, add the blank DMSO solution or sample DMSO solution, mix well, keep the temperature at 37°C for 20 minutes, add the substrate, mix well, and immediately detect the change value of the absorbance of the system within 1min at a wavele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com