A strain of lactic acid bacteria capable of absorbing or degrading enterotoxin and its application
A technology of enterotoxin and lactic acid bacteria, applied in the field of lactic acid bacteria, can solve the problems of long effective time, dependence, side effects, etc., achieve good acid and bile salt resistance, and enhance the body's immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Screening of lactic acid bacteria with the function of removing enterotoxin
[0026] 1.1 Culture of lactic acid bacteria and preparation of fermentation broth
[0027] Lactic acid bacteria were cultured in MRS liquid medium at 37°C for 24h, centrifuged at 6000r / min at 4°C for 10min, and the supernatant was collected as the fermentation broth.
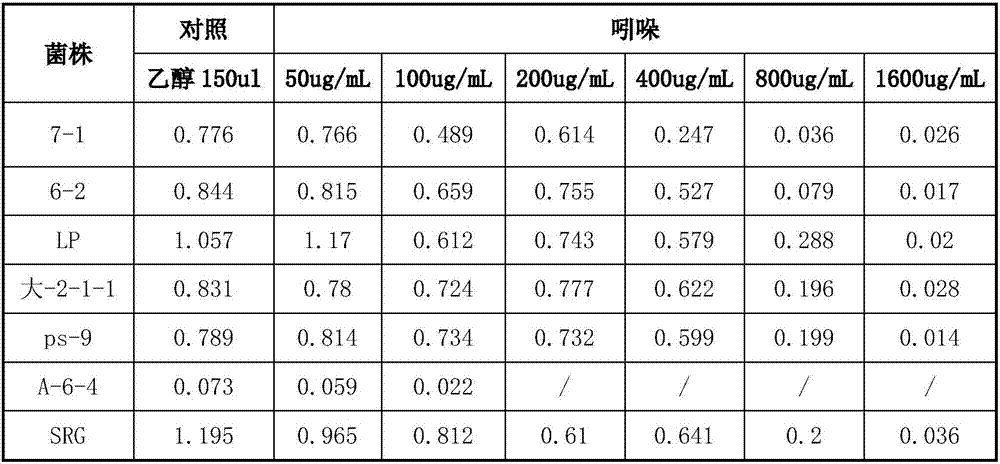
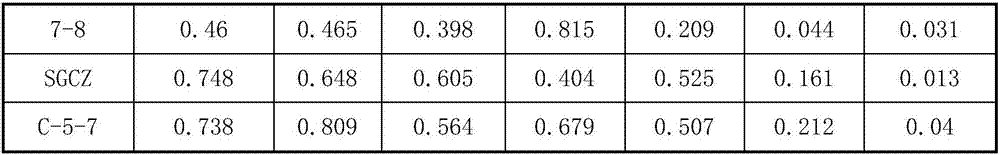
[0028] 1.2 Determination of tolerance of lactic acid bacteria in different toxins and different concentrations
[0029] Take 2mL of indole, phenol, p-cresol mother liquor and 100mL MRS medium, prepare medium with toxin concentration of 0.1, 0.1, 0.5mg / mL, inoculate lactic acid bacteria 24h fermentation broth (2%) at 12h, 24h, After 32 hours, the living bacteria count was sampled, and the survival rate of lactic acid bacteria was calculated with the culture medium fermentation liquid without toxin as a control. Preparation of mother liquor: phenol (60 degrees temperature from crystal state to liquid), p-cresol, respect...
Embodiment 2
[0032] Embodiment 2 acid resistance test
[0033] Use HCl to adjust the pH value of the normal saline to 1.5, 2.0, 3.0 respectively, sterilize at 115°C for 30 minutes, insert 1% of the inoculum into the bacterial liquid fermented for 24 hours aseptically, and incubate at 37°C for 1h, 2h, After 3 hours, samples were taken to determine the number of viable bacteria, and saline was used as a control. Survival rate (%)=the number of viable bacteria in the bacterial liquid to be tested / the number of viable bacteria in salt water×100.
Embodiment 3
[0034] Embodiment 3 bile salt resistance test
[0035] Bacterial solution was inoculated in the MRS medium with bile salt concentration of 0.1%, 0.2%, 0.3%, (w / v) by 1% (v / v), and the MRS medium without bile salt was used as a control, at 37 After culturing at ℃ for 0h, 2h, 4h, and 6h, samples were taken to count plate colonies, and the survival rate of Lactobacillus was calculated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com