Method and apparatus for producing formic esters by comprehensively utilizing metronidazole hydroxylation synthesis wastewater
A technology for the hydroxylation and chemical synthesis of metronidazole is applied in the field of comprehensive recycling and utilization of chemical synthesis pharmaceutical production wastewater, which can solve the problems of formic acid consumption and waste, difficult treatment of hydroxylation synthesis production wastewater, etc. Excellent quality of by-products and the effect of eliminating formic acid waste gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
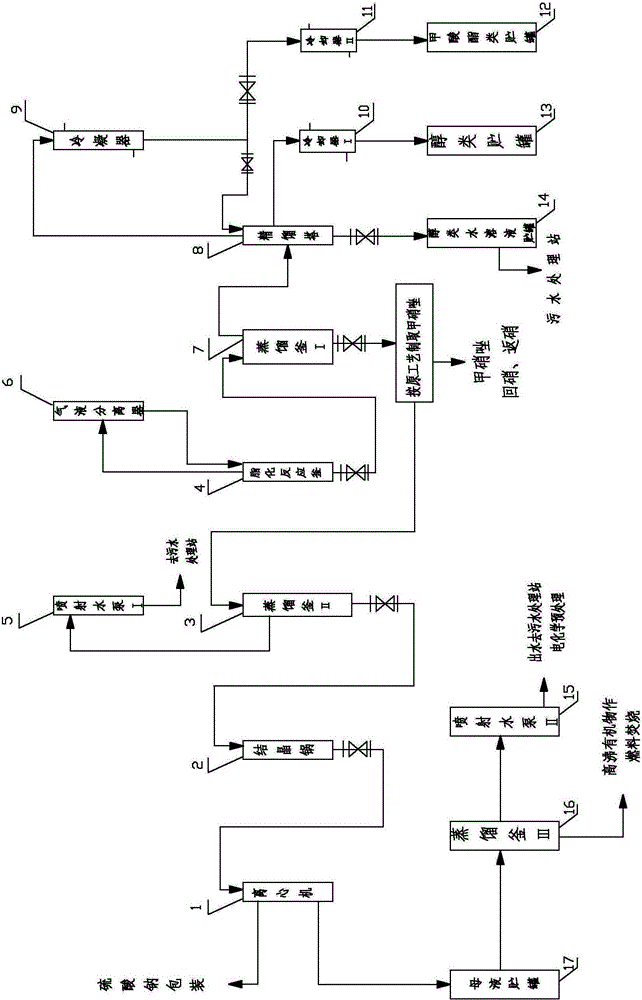
[0037] After the hydroxylation synthesis reaction is completed, the material is transferred to the esterification reactor 4 to prepare for the esterification reaction. Assuming that the quality of formic acid added when the hydroxylation synthesis reaction takes place is Q (Kg), the amount of methyl alcohol to be added is calculated as follows: W 甲醇 =1.05×Q / 46×32, then slowly add it into the esterification reaction kettle 4, after the feeding is completed, control the temperature to be greater than or equal to 60°C, reflux for 2 hours, and after the reaction is completed, transfer the material to No. Distillation, the distilled mixed gas is directly sent to the rectification tower 8 (the rectification tower is a single-tower process) for rectification separation, and the distillation operation temperature is controlled at 88 ± 2°C until all the methyl formate and methanol are distilled out and sent to enter the rectification tower for rectification separation, obtain methyl fo...
Embodiment 2
[0042] The technological process of embodiment 2 is with embodiment 1.
[0043] The difference is that:
[0044] (1) The alcohols that join in the esterification reactor 4 are ethanol, and the esterification product that obtains is ethyl formate.
[0045] (2) Ethyl formate and water have an azeotropic point (54.23°C), and the rectification process must adopt a double-tower (or three-tower) process. The double-tower process is to use the rectification tower 8 to distill ethyl formate and ethanol first, and then use The dehydration of the desiccant skips the azeotropic point, and then continues the rectification and separation in the rectification tower 8, and intercepts the fraction at 54±0.5°C at the top of the tower to obtain more than 99% of the ethyl formate product; what is obtained at the bottom of the tower is ethanol And the distillate entrainment composition when the previous process evaporates, discharges to the ethanol aqueous solution storage tank 14, and then send...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com