Preparation method and application of acellular matrix biological material
An acellular matrix and biomaterial technology, which is applied in the field of the preparation of acellular matrix biomaterials and can solve problems such as uncertain clinical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
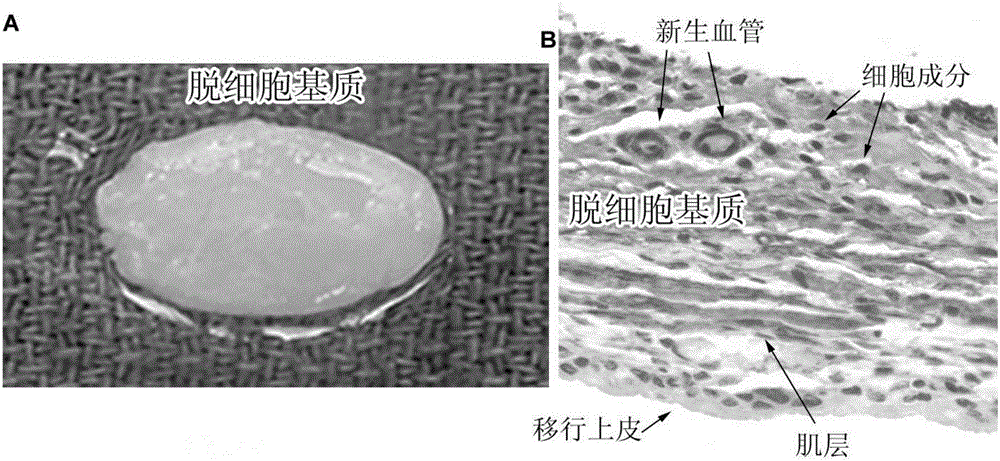
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of dermis-based acellular matrix material:
[0047] 1) The skin is taken from the skin of the above-mentioned animals, which can be taken from dead bodies and excess skin from surgical procedures;
[0048] 2) Separate the epidermis and dermis: soak the skin in 0.25% trypsin and 1mM EDTA solution for 3 hours to digest;
[0049] 3) The epidermis is separated from the dermis by mechanical means;
[0050] 4) decellularization treatment;
[0051] 5) Decellularization solution preparation: 0.125% trypsin, 1mM EDTA, 0.25% Triton X-100;
[0052] 6) Soak the corium obtained above in the decellularized solution and digest it with shaking at 37 degrees for 3-4 hours;
[0053] 7) Rinse with PBS, and then soak in phosphate-buffered saline (PBS) containing 0.1% sodium azide for several minutes to several days;
[0054] 8) Sterilization treatment: the obtained dermal matrix is irradiated with gamma rays to achieve the purpose of sterility.
Embodiment 2
[0055]Example 2: Preparation of bladder-based acellular matrix material:
[0056] 1) Place the bladder tissue in a petri dish, add 50ml of PBS containing 0.1% sodium azide, and scrape off the mucosal layer with a glass slide;
[0057] 2) The remaining muscle layer and submucosa continued to remain in the above solution for 5 hours to dissolve the cells;
[0058] 3) Rinse the bladder tissue with 50ml PBS;
[0059] 4) DNase digestion: digest the bladder with 50 ml of 1 M sodium chloride solution containing 2000 units of DNase (Sigma Company) for 12 hours. Then remove the solution, add new digestion solution and repeat 3 times;
[0060] 5) Then treat with 50 ml of 4% sodium deoxycholate (sodium deoxycholatel) containing 0.1% sodium azide for 6 hours and stir, and repeat this process a total of 3 times;
[0061] 6) The obtained acellular matrix is kept at 4°C in a solution containing 0.1% sodium azide and 10% neomycin sulfate;
[0062] 7) Sterilization treatment: the obtaine...
Embodiment 3
[0063] Embodiment 3: adipose stem cell is free:
[0064] 1) Adipose tissue or fat obtained by liposuction;
[0065] 2) Rinse with PBS containing 1% penicillin and streptomycin;
[0066] 3) Cut the tissue into small pieces of 1 mm square with a blade;
[0067] 4) Add 0.075% collagenase type 1A (collagenase type IA, Sigma company) at the ratio of solution:tissue 5:1;
[0068] 5) Incubate with a shaker at 37 degrees for 1 hour;
[0069] 6) Centrifuge at 250 g for 10 minutes at room temperature. At this time, the solution is divided into three layers, and the lower layer is the digested cell pellet;
[0070] 7) Remove the upper two layers and keep the cell layer;
[0071] 8) Resuspend the cells in adipose tissue maintenance solution containing 200mg / ml KH2PO4, 200mg / L KCl, 2.16g / L Na 2 HPO 4 .7H 2 O, 8g / L NaCl, 30,000 units / L SOD, 5g / L human serum albumin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com