Iron-based catalyst and preparation method thereof
A catalyst and catalyst carrier technology, applied in the field of coal chemical industry, can solve the problems of poor catalytic activity, poor dispersion and stability of iron-based catalysts, etc., and achieve the effects of improving catalytic activity, improving stability and high coal direct liquefaction activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
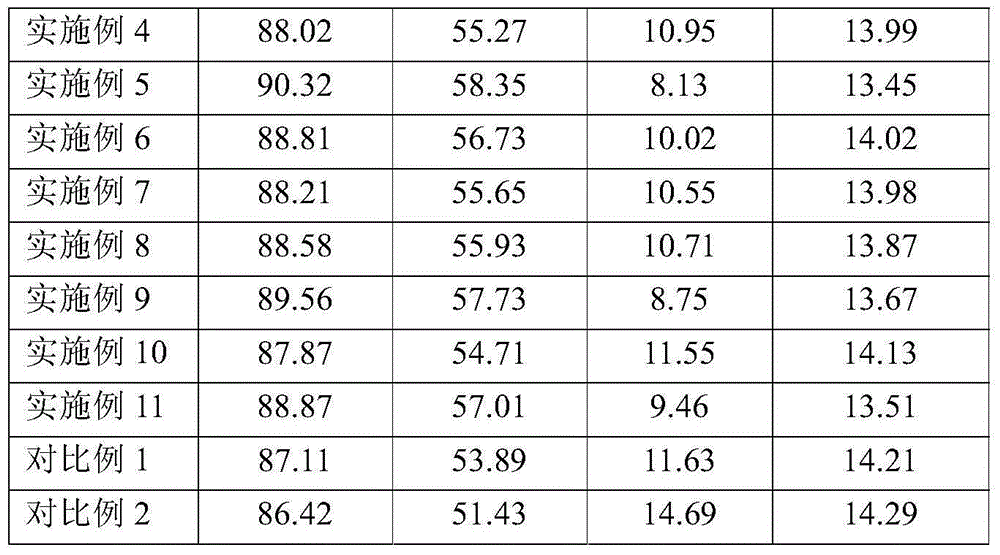
Examples
preparation example Construction
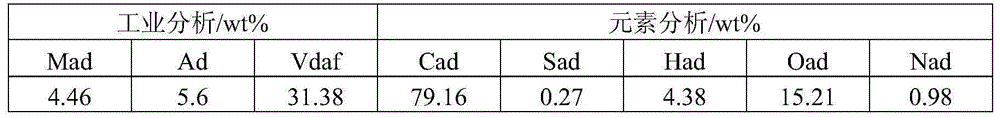
[0031] In the above preparation method, those skilled in the art can choose the specific operation method of each step. In a preferred embodiment, the pulping step includes: dissolving iron-containing soluble salts and manganese-containing soluble salts in a first solvent to form a first solution; and performing an oxidation reaction on the first solution with an alkaline solution to obtain Slurry; wherein, the first solvent is water, and the alkaline solution includes but not limited to ammonia water or an aqueous solution of inorganic alkali.
[0032] The above-mentioned principle of preparing the iron-based catalyst of the present invention is to dissolve the iron-containing soluble salt and the manganese-containing soluble salt in the first solvent to form the first solution; the first solution is mixed with the alkaline solution, and a precipitation reaction occurs first, and simultaneously Compressed air is passed through the above reaction liquid, and oxidation reaction...
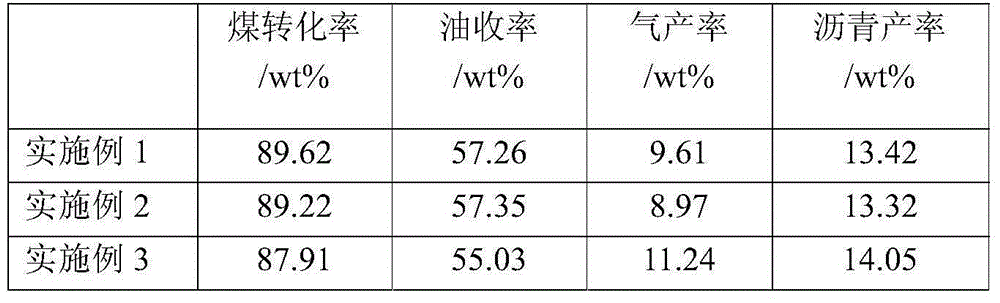
Embodiment 1
[0041] Weigh 74.5gFeSO 4 ·7H 2 O was added to 600g deionized water, stirred and dissolved, and 0.61gMnSO was added 4 ·H 2 O in ferrous sulfate solution, form the mixed solution of ferrous sulfate containing manganese; Prepare the ammoniacal solution of 500g2.5wt%. Feed the above-mentioned manganese-containing ferrous sulfate solution and ammonia solution in parallel to cause precipitation reaction of ferrous iron. At the same time, air is introduced to carry out oxidation reaction. The reaction time is 60 minutes. During this period, the feed rate of ammonia water is controlled to maintain the pH value of the reaction solution. is 8.0. After the reaction, FeOOH and MnO(OH) are obtained 2 of mixed slurry.
[0042] Add 400 g of dry coal powder with a particle size of less than 200 μm to the above slurry, stir well and evenly, and filter to obtain a filter cake. Wash the filter cake with deionized water until the measured conductivity of the filtrate is less than 1000 μs / cm...
Embodiment 2
[0044] Weigh 348gFeSO 4 ·7H 2 O was added to 1200g of deionized water, stirred and dissolved, and 27.65g of MnSO was added 4 ·H 2 O in ferrous sulfate solution, form the mixed solution of ferrous sulfate containing manganese; Prepare the aqueous ammonia solution of 2150g2.5wt%. The above-mentioned manganese-containing ferrous sulfate solution and ammonia solution are fed in parallel to cause precipitation reaction of ferrous iron, and at the same time, air is introduced for oxidation. The reaction time is 80 minutes. During this period, the feed rate of ammonia water is controlled to maintain the pH value of the reaction solution. 8.0. After the reaction is completed, the reaction including FeOOH and MnO(OH) 2 of mixed slurry.
[0045] Add 300 g of dry coal powder with a particle size of less than 200 μm to the above slurry, stir well and evenly, and filter to obtain a filter cake. Wash the filter cake with deionized water until the measured conductivity of the filtrate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com