Moxifloxacin hydrochloride film-coated tablet and preparation method thereof
A technology of moxifloxacin hydrochloride and film-coated tablets, applied in sugar-coated pills, pill delivery, antibacterial drugs and other directions, can solve the problems of poor water solubility, affected safety, difficult to granulate, etc., and achieves easy operation and pharmaceutical excipients. Few, simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Moxifloxacin hydrochloride film-coated tablet
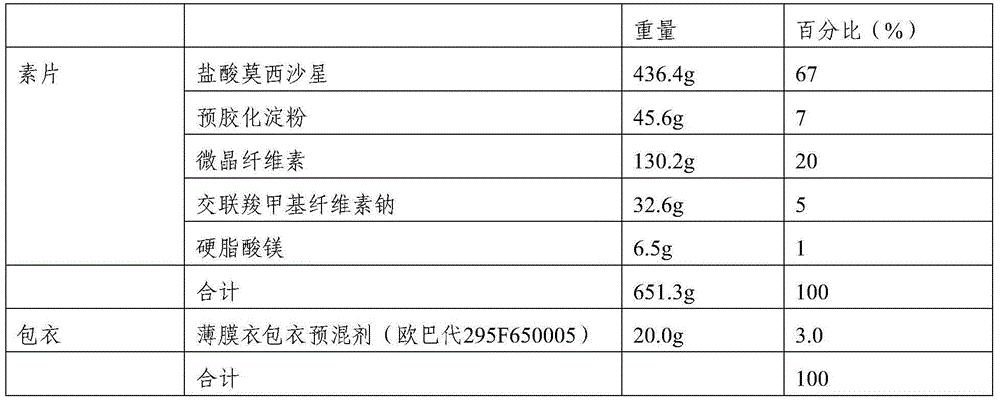
[0035] 1. Composition: The film-coated tablet is calculated by weight percentage, and the prescription of 1000 tablets (calculated on the basis of uncoated tablet) is as follows:
[0036]
[0037] 2. Preparation process:
[0038] (1) The raw and auxiliary materials are processed through an 80-mesh sieve;
[0039] (2) Wet mixing and granulation: Moxifloxacin hydrochloride, microcrystalline cellulose, pregelatinized starch, and croscarmellose sodium that have been sieved are put into a wet granulator and stirred at high speed, and microcrystalline Purified water with twice the volume of the cellulose prescription is used to make wet granules with a 20-mesh screen in a swing granulator, and the wet granules are dried in a hot air circulation oven at 55°C until the moisture is less than 3%;
[0040] (3) Tablet compression: dry granules are mixed with sieved magnesium stearate, put into a motion mixer for mix...
Embodiment 2
[0042] Embodiment 2: Moxifloxacin hydrochloride film-coated tablet
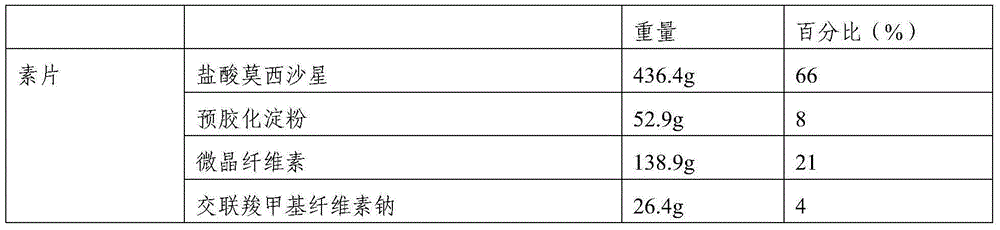
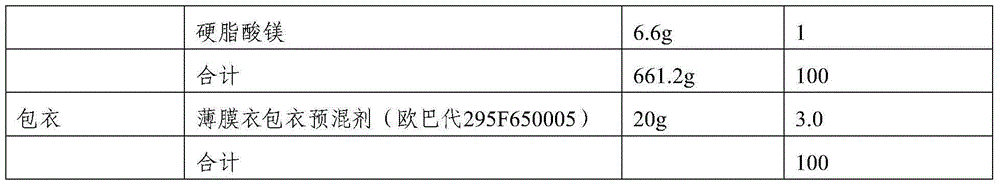
[0043] 1. Composition: The film-coated tablet is calculated by weight percentage, and the prescription of 1000 tablets (calculated on the basis of uncoated tablet) is as follows:
[0044]
[0045]
[0046] 2. Preparation process:
[0047] (1) The raw and auxiliary materials are processed through an 80-mesh sieve;
[0048] (2) Wet mixing and granulation: Moxifloxacin hydrochloride, microcrystalline cellulose, pregelatinized starch, and croscarmellose sodium that have been sieved are put into a wet granulator and stirred at high speed, and microcrystalline Purified water with twice the volume of the cellulose prescription is used to make wet granules with a 20-mesh screen in a swing granulator, and the wet granules are dried in a hot air circulation oven at 50°C until the moisture is less than 4%;
[0049] (3) Tablet compression: dry granules are mixed with sieved magnesium stearate, put into a motion mix...
Embodiment 3
[0051] Embodiment 3: Moxifloxacin hydrochloride film-coated tablet
[0052] 1. Composition: The film-coated tablet is calculated by weight percentage, and the prescription of 1000 tablets (calculated on the basis of uncoated tablet) is as follows:
[0053]
[0054] 2. Preparation process:
[0055] (1) The raw and auxiliary materials are processed through an 80-mesh sieve;
[0056] (2) Wet mixing and granulation: Moxifloxacin hydrochloride, microcrystalline cellulose, pregelatinized starch, and croscarmellose sodium that have been sieved are put into a wet granulator and stirred at high speed, and microcrystalline Purified water with twice the volume of the cellulose prescription is used to make wet granules with a 20-mesh screen in a swing granulator, and the wet granules are dried in a hot air circulation oven at 60°C until the moisture is less than 2%;
[0057] (3) Tablet compression: dry granules are mixed with sieved magnesium stearate, put into a motion mixer for mix...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com