A compound liver-protecting traditional Chinese medicine for treating non-alcoholic fatty liver
A non-alcoholic and fatty liver technology, applied in the field of compound liver-protecting traditional Chinese medicine for the treatment of non-alcoholic fatty liver, can solve the problems of reducing absorption, increasing carotenoid absorption, etc. Effects of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1, In Vitro Experiment of the Compound Liver-Protecting Traditional Chinese Medicine in Treating Nonalcoholic Fatty Liver
[0040] 1. Liquid preparation
[0041] (1) Preparation of 1% (w / w) PBS buffer
[0042] Weigh 2.0g of PBS powder into a 250mL jar, add 200mL of double-distilled water to dissolve, sterilize by high-pressure steam, and store in an ordinary refrigerator at 4°C.
[0043] (2) Preparation of cell culture medium (10% of fetal bovine serum and 1% of penicillin and streptomycin)
[0044] In the ultra-clean bench, add 15 mL of fetal bovine serum to a jar (250 mL), then add 1.5 mL of double antibody (penicillin and streptomycin), and finally add an appropriate amount of RPMI-1640 culture solution to make the volume to 150 mL, filter and sterilize, Store in a normal refrigerator at 4°C.
[0045] (3) Preparation of cell cryopreservation solution
[0046] Add 7mL of RPMI-1640 culture medium, 2mL of fetal bovine serum and 1mL of DMSO into a centrifuge ...
Embodiment 2
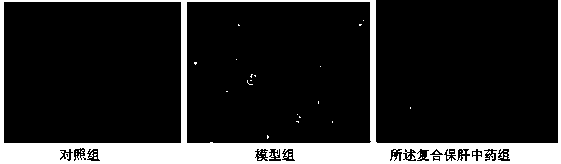
[0098] Example 2, In Vivo Experiment of the Compound Liver-Protecting Traditional Chinese Medicine in Treating Non-alcoholic Fatty Liver
[0099] 1. Experimental materials
[0100] 1.1 Experimental animals
[0101] C57BL / 6J male mice were purchased from Beijing Weitong Lihua Company, SPF grade, weighing 18-22 g. The compound liver-protecting traditional Chinese medicine was purchased from Dalian Zhongke Geleike Biotechnology Co., Ltd. The experimental animals were kept under SPF standard laboratory conditions, kept well ventilated, the ambient temperature was controlled at 23°C±2°C, the humidity was controlled at 40%-50%, 12h light / 12h dark cycle, and free intake of food and water. The mice were fed normally for 1 week before the start of the study, and the experiment started from the 2nd week.
[0102] 1.2 Feed formula
[0103] 10% of the energy in the basic feed comes from fat, 70% from carbohydrates, and 20% from protein. 45% of the energy in a high-fat diet comes from f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com