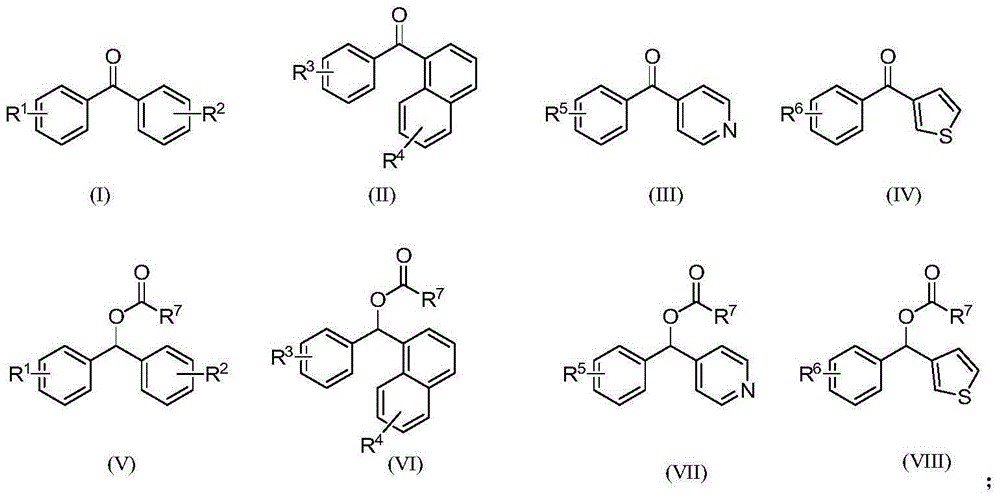
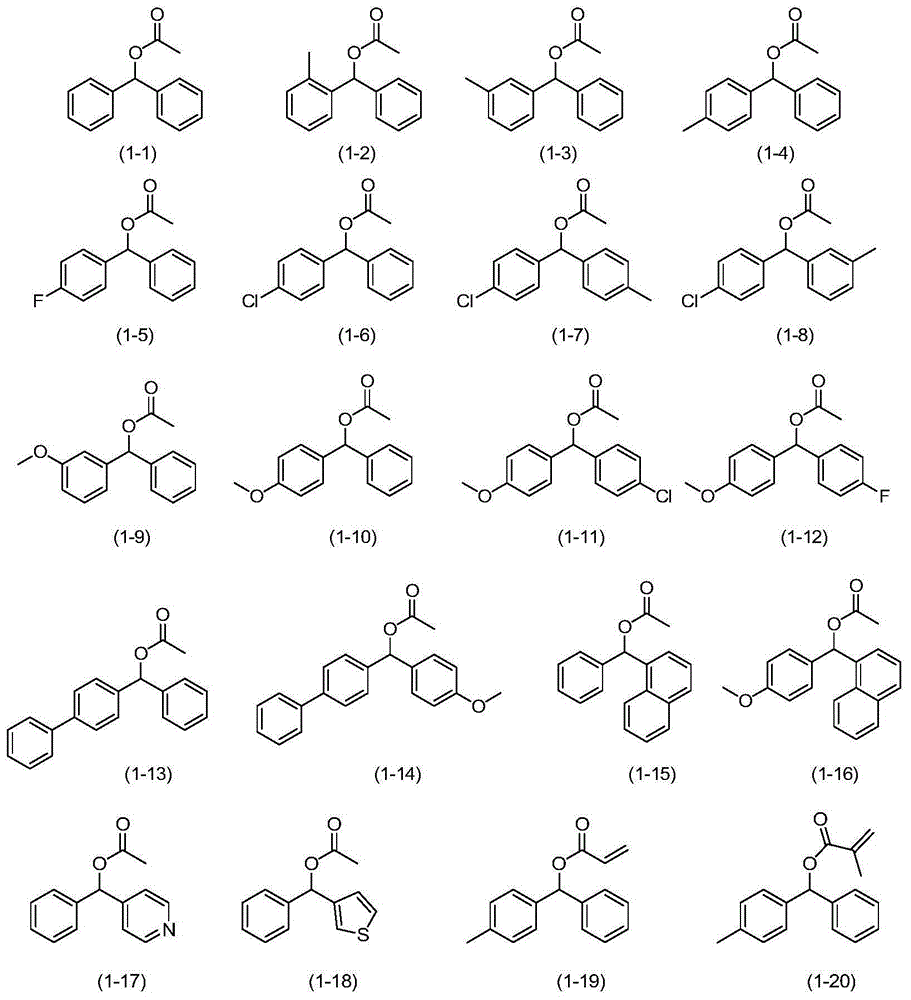
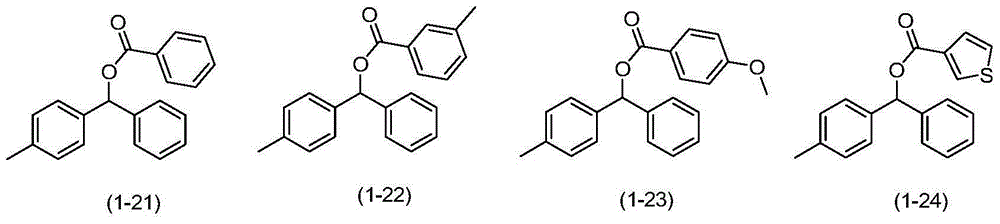
Synthetic method for diaryl ketone compounds
A technology for diaryl ketones and synthesis methods, which is applied in the field of synthesis of diaryl ketones, can solve problems such as complicated operations, equipment corrosion, and difficult separations, and achieve high product selectivity, mild reaction conditions, and simplified The effect of the action steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of benzophenone (formula (2-1))
[0030] Add diphenylmethanol acetate (formula (1-1), 0.45g, 2mmol), DDQ (0.45g, 2mmol), H 2 O (0.18g, 10mmol) and chlorobenzene (8ml) were reacted with stirring at 130°C, monitored by TLC, and reacted for 2h. The reaction solution was sampled and analyzed by gas chromatography (GC). The conversion rate was 100%, and the product selectivity was 99%. The reaction liquid was evaporated to remove the solvent under reduced pressure, passed through a silica gel column, and a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:200 was used as the eluent to collect the eluate containing the target compound, evaporated to remove the solvent, and then dried to obtain two Benzophenone 0.35g, the isolated yield of benzophenone is 97%.
Embodiment 2
[0031] Embodiment 2: the preparation of benzophenone (formula (2-1))
[0032] The reaction steps were the same as in Example 1, except that chlorobenzene was changed to o-dichlorobenzene, and the reaction was stirred at 140° C. for 1.5 hours. The reaction conversion rate was 100%, and the product selectivity was 99%. Finally, 0.35 g of benzophenone was obtained, and the isolated yield of benzophenone was 97%.
Embodiment 3
[0033] Embodiment 3: the preparation of phenyl o-tolyl ketone (formula (2-2))
[0034] Add phenyl-o-tolylmethanol acetate (formula (1-2), 0.48g, 2mmol), DDQ (0.54g, 2.4mmol), H 2 O (0.18g, 10mmol) and chlorobenzene (8ml) were reacted under stirring at 130°C, monitored by TLC, and reacted for 3h. The reaction solution was sampled and analyzed by gas chromatography (GC). The conversion rate was 98%, and the product selectivity was 99%. The reaction solution was evaporated to remove the solvent under reduced pressure, passed through a silica gel column, and the mixture of ethyl acetate and petroleum ether with a volume ratio of 1:200 was used as the eluent to collect the eluate containing the target compound, evaporated to remove the solvent, and then dried to obtain benzene 0.37 g of phenyl-o-tolyl ketone, and the isolated yield of phenyl-o-tolyl ketone was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com