Hydroxyanthraquinone chlormethine derivative having antitumor activity, and preparation method thereof
A technology for hydroxyanthraquinone nitrogen mustard and antitumor activity, which is applied in the field of hydroxyanthraquinone nitrogen mustard derivatives with antitumor activity and the preparation thereof, can solve problems such as failure to provide nitrogen mustard, and achieves high selectivity and synthetic route. Simple, easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
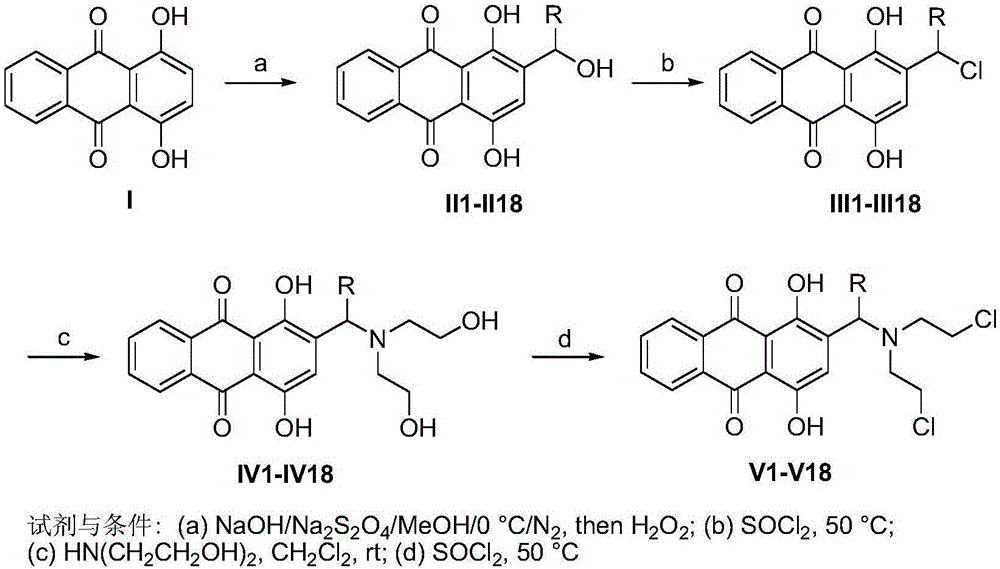
[0022] The preparation method of hydroxyanthraquinone nitrogen mustard derivatives: take 1,4-dihydroxyanthraquinone as raw material, through 2-hydroxyalkylation, then 2-hydroxyalkyl chlorination, and then link with diethanolamine, finally will get The complex is chlorinated with thionyl chloride to obtain the target compound; the specific method is:
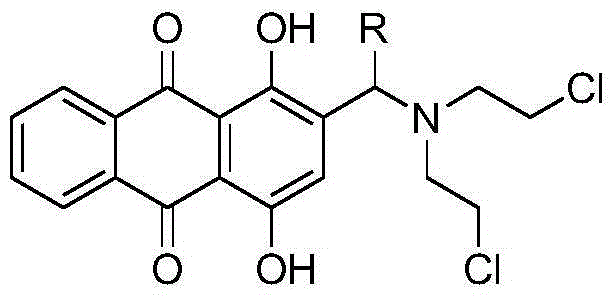
[0023] Using 1,4-dihydroxyanthraquinone as a raw material, introduce a hydroxyalkyl group at the 2-position of 1,4-dihydroxyanthraquinone through hydroxyalkylation to obtain 2-(1-substituted-1-hydroxymethyl)-1, 4-dihydroxy-9,10-anthraquinone, and then react with thionyl chloride to chlorinate the hydroxyalkyl to obtain 2-(1-substituted-1-chloromethyl)-1,4-dihydroxy-9,10 -anthraquinone, then react with diethanolamine at room temperature, and finally the resulting 2-(1-substituted-bis(2-hydroxyethylamino)methyl)-1,4-dihydroxy-9,10-anthraquinone React with thionyl chloride to obtain 2-(1-substituted-bis(2-chloroethylamino)methyl)-1...
Embodiment 1
[0033] Example 1: 2-(1-substituted-1-hydroxymethyl)-1,4-dihydroxy-9,10-anthraquinone II1-II18 synthetic general method:
[0034] 1,4-Dihydroxyanthraquinone I (1mmol) was dissolved in methanol (20ml), sodium hydroxide solution (1N, 6ml) was added, and sodium dithionite (2mmol) dissolved in water (2mL) was added under nitrogen. After stirring for 10 minutes, aldehyde (2 mmol) was added and reacted at 0° C. for 3 hours. The reaction solution was poured into 10 mL of ice water added with 30% hydrogen peroxide (2 mL), and stirred for 10 minutes. Add hydrochloric acid solution (3N, 2mL) to acidify, extract with dichloromethane, wash with saturated sodium bicarbonate solution, water, and saturated brine, and dry over anhydrous magnesium sulfate. Separation by column chromatography (dichloromethane) gave orange-red solids II1-II18.
[0035] 2-Hydroxymethyl-1,4-dihydroxy-9,10-anthraquinone II1
[0036] Yield 44%; mp: 208-209°C; 1 HNMR (400MHz, DMSO-d 6)δ13.09(s,1H),12.81(s,1H),8.2...
Embodiment 2
[0071] Example 2: General Synthesis of 2-(1-substituted-1-chloromethyl)-1,4-dihydroxy-9,10-anthraquinone III1-III18:
[0072] Thionyl chloride (1.0 ml) was added to 2-(1-substituted-1-hydroxymethyl)-1,4-dihydroxy-9,10-anthraquinone II (1 mmol). After reacting at 50°C for 2 hours, excess thionyl chloride was distilled off under reduced pressure. The crude product was used directly in the next step without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com