Recombinant vector containing Jerusalem artichoke fructan 1-exohydrolase gene and application thereof in producing alcohol by fermentation
A kind of technology of hydrolase and fructan, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
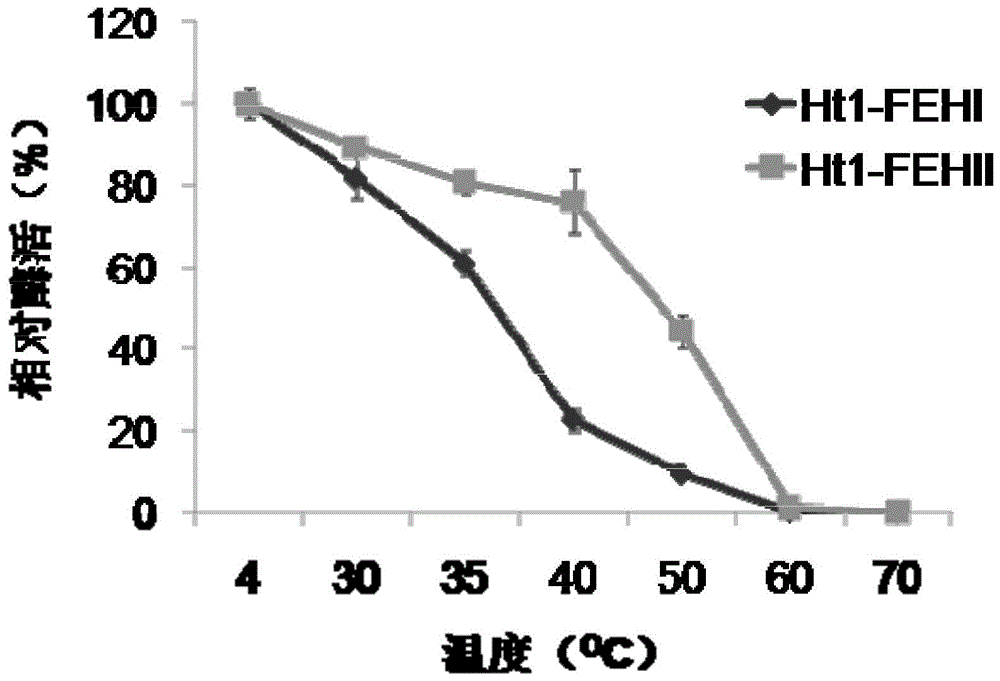
[0035] The thermal stability of the Jerusalem artichoke Ht1-FEHs of embodiment 1 recombination
[0036] The temperature stability of the purified recombinant Ht1-FEHs enzyme was determined by putting the recombinant enzyme in a water bath at different temperatures (4, 30, 35, 40, 50, 60, 70°C) for 2 hours, then placing the recombinant enzyme on ice to restore the low temperature, and then Mix with the most suitable substrate 1-kestose, pH 6, react overnight at 35°C, and determine the remaining enzyme activity. The temperature stability of the enzyme was determined by taking the enzyme activity of the recombinant enzyme at 4°C as a reference.
[0037] The result is as figure 1 As shown, the purified recombinant enzyme Ht1-FEHII still maintains more than 80% of its activity after being incubated at a temperature below 40°C for 2 hours, indicating that the enzyme is very stable at room temperature, while the enzyme activity of Ht1-FEHI at a temperature above 35°C will decrease. ...
Embodiment 2
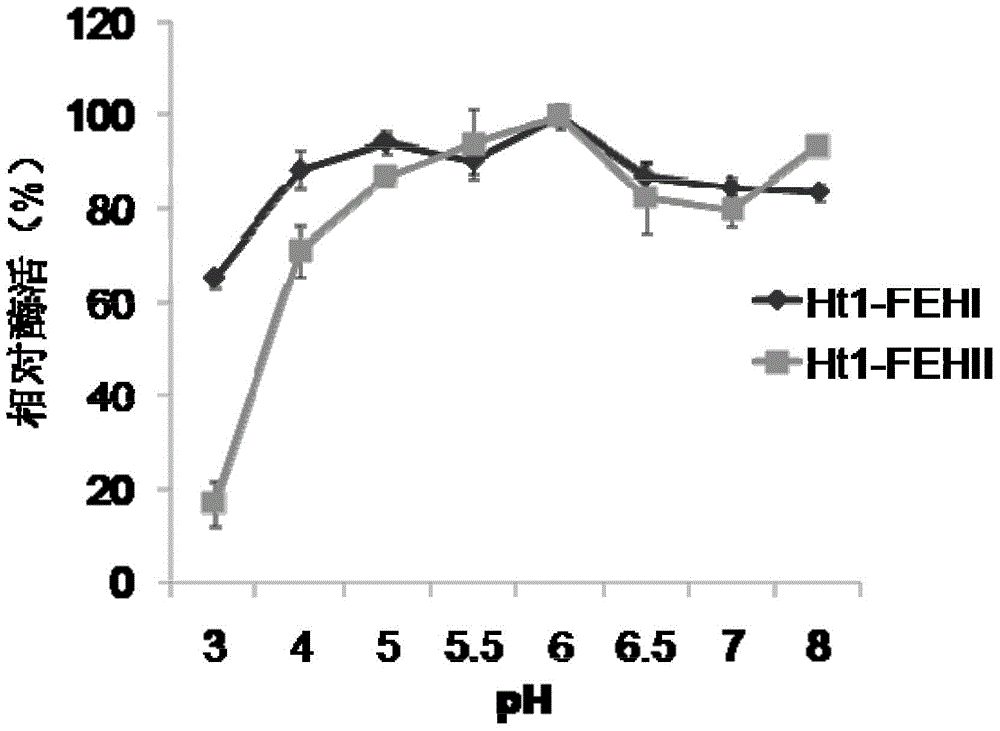
[0038] The pH stability of the recombinant Jerusalem artichoke Ht1-FEHs of embodiment 2
[0039] Mix the purified recombinant proteins Ht1-FEHI and II with buffers of different pH (3, 4, 5, 5.5, 6, 6.5, 7, 8), and measure the remaining enzyme activity after standing at 0°C for 24 hours to evaluate the recombinant Enzyme stability to pH. The pH stability of inulinase was determined with reference to the enzyme activity placed at the optimum pH6. Method for measuring remaining enzyme activity: 1-kestose was used as substrate, pH 6, reacted overnight at 35°C, and the change of enzyme activity under the influence of different pH was measured. The results show (such as figure 2 ), the enzyme activities of the recombinant proteins Ht1-FEHI and II were relatively stable between pH 4 and 8.
Embodiment 3
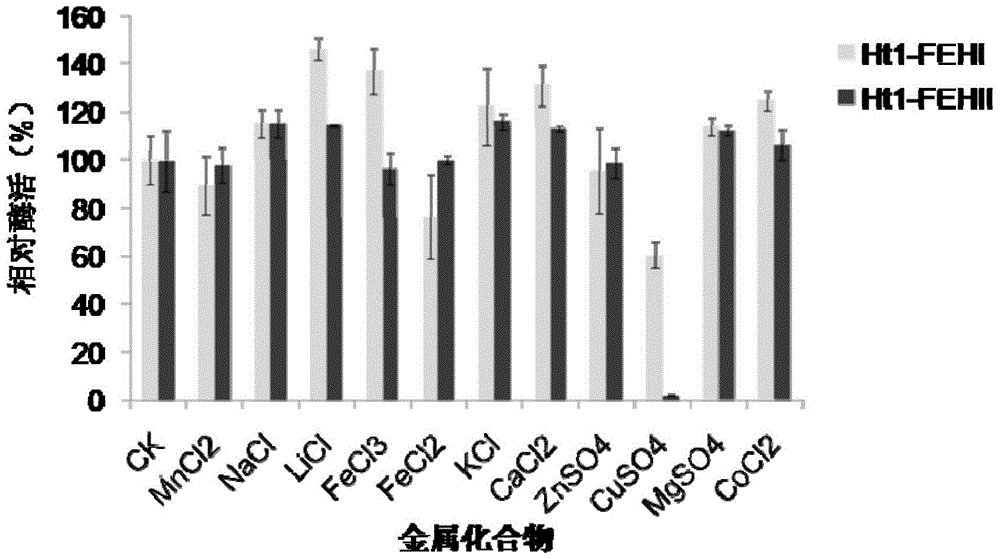
[0040] The effect of different metal compounds of embodiment 3 on the activity of recombinant Jerusalem artichoke Ht1-FEHs
[0041] Mix the purified recombinant proteins Ht1-FEHI and II with different metal compounds, the final ion concentration is 1.0mM, and place it at 0°C for 60min to measure the inulinase activity. The metal ions include ZnSO 4 , CuSO 4 .5H 2 O, MgSO 4 .5H 2 O, FeCl 3 , CaCl 2 , KCl, MnCl 2 , LiCl, FeCl 2 , NaCl and CoCl 2 etc., the control is the enzyme activity of the recombinant enzyme added with the same volume of distilled water, and the influence of metal ions on the enzyme activity is judged according to the remaining enzyme activity.
[0042] The result is as image 3 As shown, 1.0mM Li + , Ca 2+ 、K + 、Na + , Mg 2+ and Co 2+ There are different degrees of activation of recombinase Ht1-FEHI and II, while Fe 2+ and Cu 2+ It has a certain inhibitory effect on the enzyme activity of recombinase 1-FEHs, indicating that they can change t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com