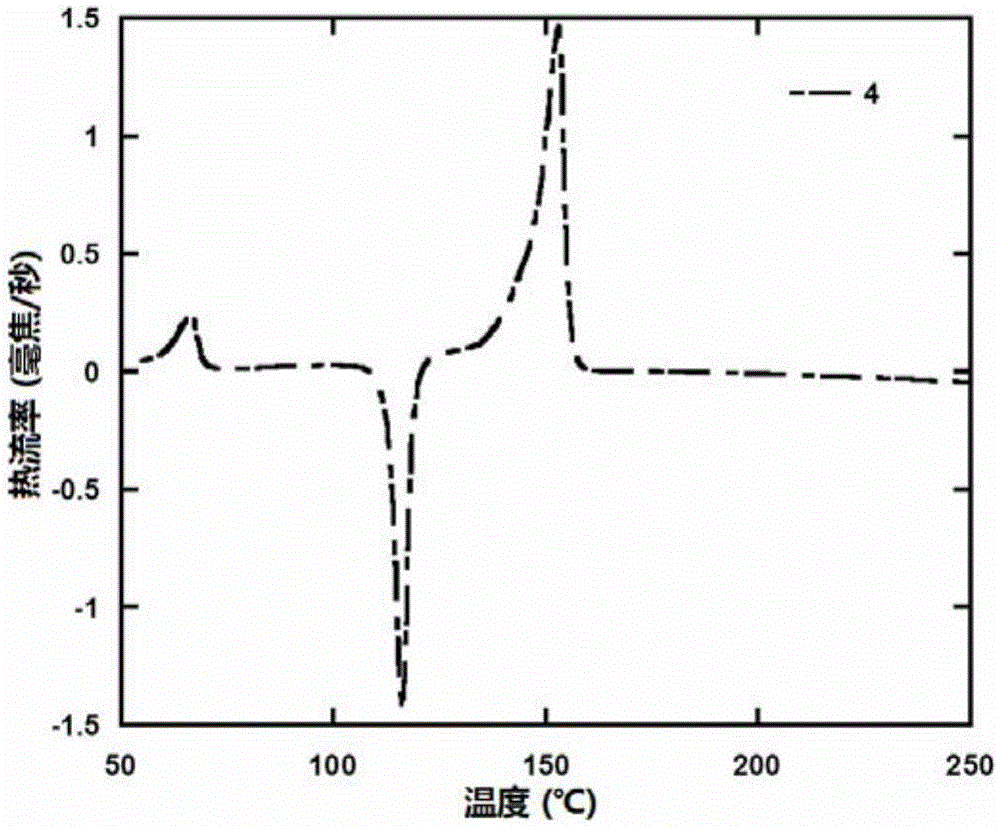
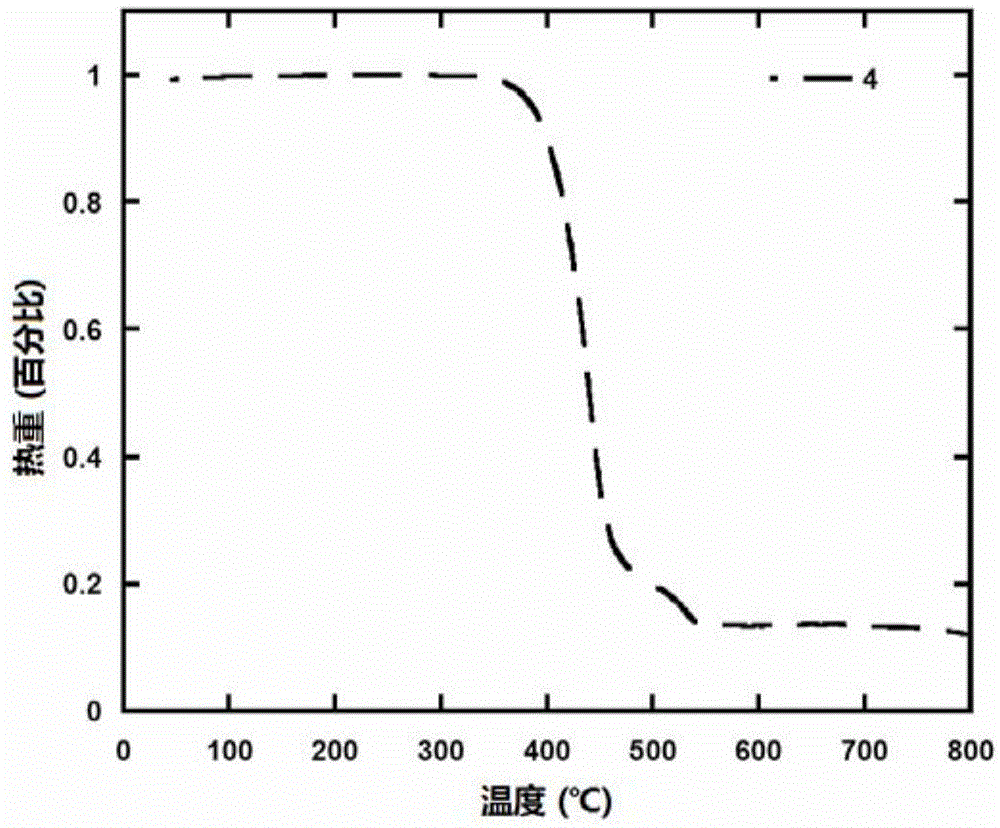
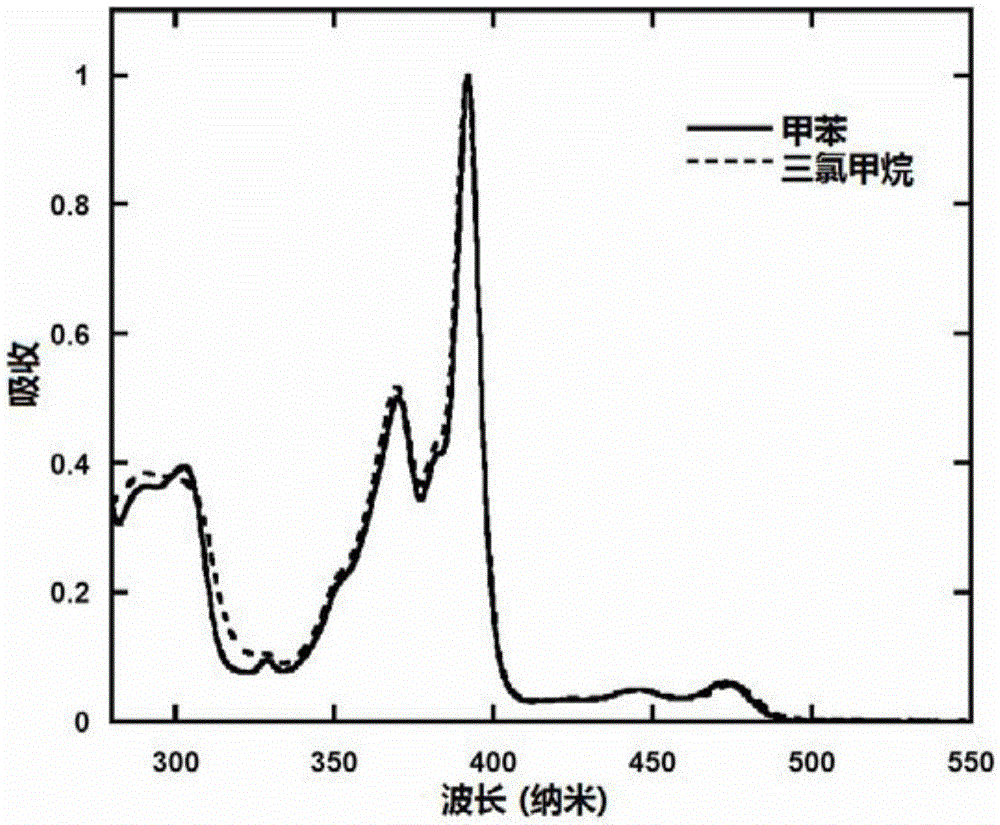
Nine-membered fused ring derivatives as well as synthesis method and application thereof
A derivative and condensed ring technology, applied in the field of organic optoelectronics, can solve problems such as increasing device stability, and achieve the effects of low cost, increased conjugation, and high mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0035] Synthesis of embodiment 13,9-dibromo-5,11-dihexadecyl indolecarbazole (1)
[0036]
[0037] Dissolve 3,9-dibromoindolecarbazole (1.0g, 2.4mmol) in a 250mL three-neck flask filled with 40mL dimethyl sulfoxide, and simultaneously add 50% potassium hydroxide solution (1.4mL) and tetrabutylammonium bromide (77mg, 0.24mmol), stirred under nitrogen atmosphere for 30min, then added hexadecyl bromide (2.2g, 7.2mmol). The reaction was warmed to 65°C and reacted for 4h. The reaction was then cooled to room temperature and poured into 300 mL of methanol with stirring. After suction filtration and washing with N,N-dimethylformamide, methanol and acetone for 1-3 times, 1.5 g of a light yellow powdery solid was finally obtained, with a yield of 72%.
Embodiment 23
[0038] Example 23, 9-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,11-dihexadecylindolecarbazole (2) Synthesis
[0039]
[0040] 3,9-dibromo-5,11-dihexadecyl indolecarbazole (1.0g, 1.1mmol), potassium acetate (1.08g, 11mmol) and bispinacol borate (0.9g, 3.5 mmol) was dissolved in a 100 mL three-necked round-bottomed flask filled with 50 mL of dioxane and stirred in a nitrogen atmosphere. When the temperature rose to 80°C, 1,1'-bisdiphenylphosphinoferrocenepalladium dichloride (40mg, 0.055mmol) was added rapidly. After 24 hours of reaction, it was cooled to room temperature and filtered with suction. The initial product of the filtrate was chromatographed on a silica gel / dichloromethane column to obtain 0.7 g of yellow needle-like crystals, with a yield of 63%.
Embodiment 33
[0041] Example 33, Synthesis of 9-bis(2-methylsulfinylphenyl)-5,11-dihexadecyl indolecarbazole (3)
[0042]
[0043] 10 mL of 2M K 2 CO 3Solution, 3,9-Bis(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-5,11-Dihexadecylindolecarbazole (2.0g, 2.1mmol) and 2-bromo(methylsulfinyl)benzene (1.42g, 6.5mmol) were dissolved in 100mL of toluene solution, while adding tetrakis(triphenylphosphine)palladium (100mg) and tetrabutylbromide Ammonium chloride (60mg), the reaction was heated to reflux for 24h. The reaction was then cooled to room temperature and extracted with dichloromethane. The organic phase was dried, distilled under reduced pressure and subjected to column chromatography using silica gel, ethyl acetate / petroleum ether (volume ratio 1:2) as the mobile phase to obtain a yellow viscous oily liquid (1.25 g, 61%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com