3-(4-carbazole-9-yl-phenyl)-1-ferrocenyl-acetone and preparing method thereof
A ferrocene-based, -1- technology, applied in chemical instruments and methods, metallocene, organic chemistry, etc., can solve the problems of high equipment requirements, time-consuming and labor-consuming, complicated operation, etc., and achieve high reactivity, shorten The effect of response time and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Add 0.35 mmol acetylferrocene, 0.42 mmol 4-carbazol-9-yl-benzaldehyde, 0.42 mmol NaOH and 0.42 mmol K to a dry mortar 2 CO 3 , grind at room temperature for 15 minutes until uniform, to obtain a mixture;
[0034] 2) The mixture was incubated and reacted for 30 minutes at a temperature of 80°C. After the mixture was naturally cooled, it was washed with ethanol with a volume concentration of 95%, suction filtered, and the filter cake was recrystallized with absolute ethanol to obtain 3-(4- Carbazol-9-yl-phenyl)-1-ferrocenyl-acetone, the yield reached 97.5%.
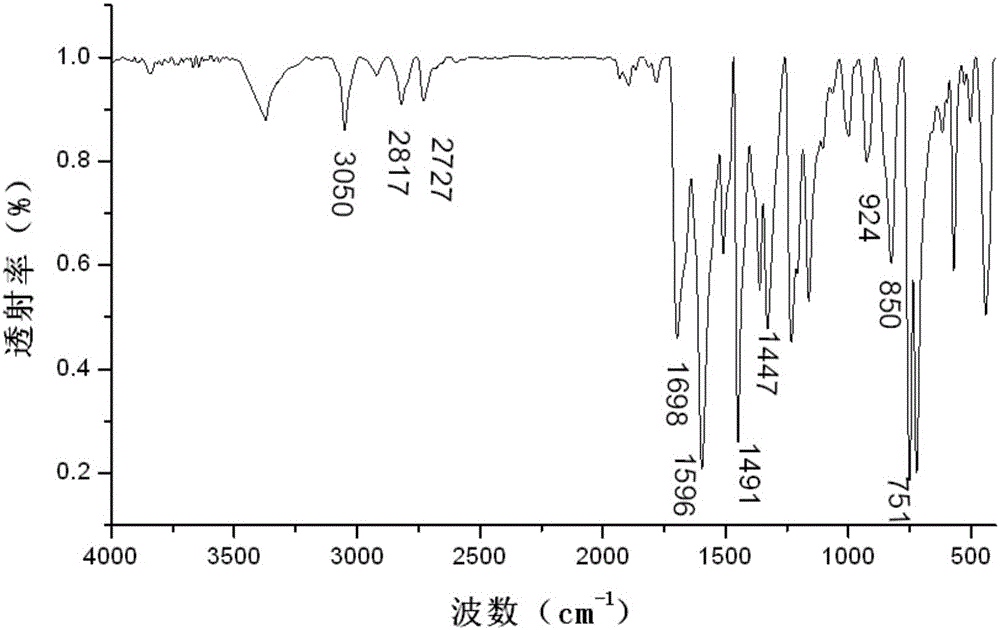
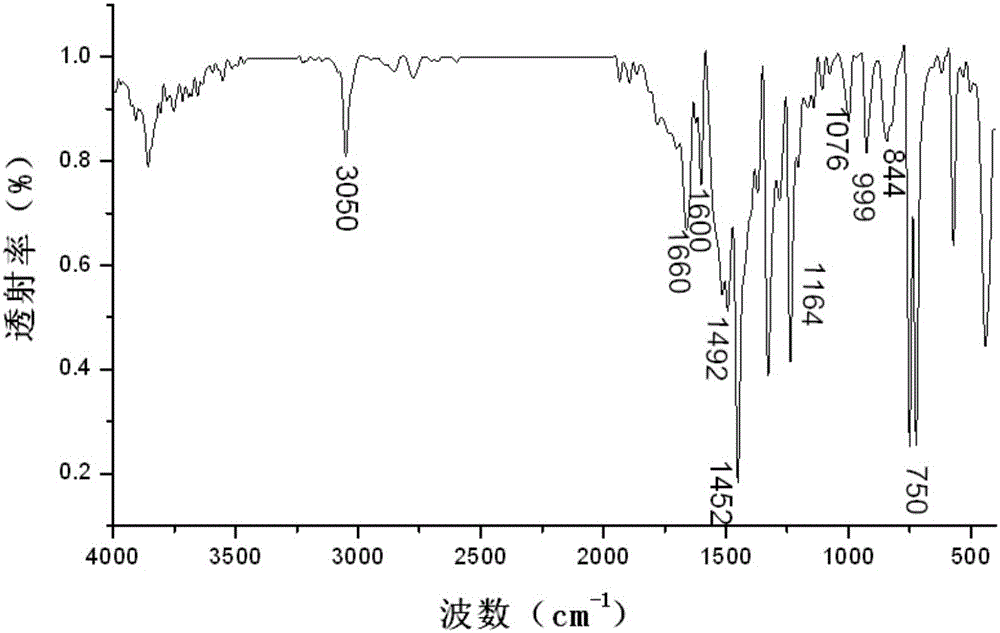
[0035] image 3 For the infrared spectrum of the obtained 3-(4-carbazol-9-yl-phenyl)-1-ferrocenyl-acetone, from image 3 As can be seen in the 3050cm -1 The stronger absorption vibration peaks are the stretching vibration absorption peak of benzene ring C-H and the vibration absorption peak of H on the unsaturated C=C double bond; at 1660cm -1 The left and right are the vibrational absorption peaks of C=O; du...
Embodiment 2
[0038] 1) Add 0.35 mmol acetylferrocene, 0.42 mmol 4-carbazol-9-yl-benzaldehyde, 0.385 mmol NaOH and 0.385 mmol K to a dry mortar 2 CO 3 , grind at room temperature for 10 minutes until uniform, to obtain a mixture;
[0039] 2) The mixture was incubated and reacted for 20 minutes at a temperature of 85°C. After the mixture was naturally cooled, it was washed with ethanol with a volume concentration of 92%, suction filtered, and the filter cake was recrystallized with absolute ethanol to obtain 3-(4- Carbazol-9-yl-phenyl)-1-ferrocenyl-acetone in a yield of 97%.
Embodiment 3
[0041] 1) Add 0.35 mmol acetylferrocene, 0.42 mmol 4-carbazol-9-yl-benzaldehyde, 0.40 mmol NaOH and 0.40 mmol K to a dry mortar 2 CO 3 , grind at room temperature for 20 minutes until uniform, to obtain a mixture;
[0042] 2) The mixture was incubated and reacted for 40 minutes at a temperature of 82°C. After the mixture was naturally cooled, it was washed with ethanol with a volume concentration of 90%, suction filtered, and the filter cake was recrystallized with absolute ethanol to obtain 3-(4- Carbazol-9-yl-phenyl)-1-ferrocenyl-acetone, the yield reached 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com