Recombinant human/baboon chimeric uricase protein and preparation method thereof
A uricase and protein technology, applied in the field of medicine, can solve the problem of less than 40% homology, achieve the effects of reducing immunogenicity, reasonable design, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
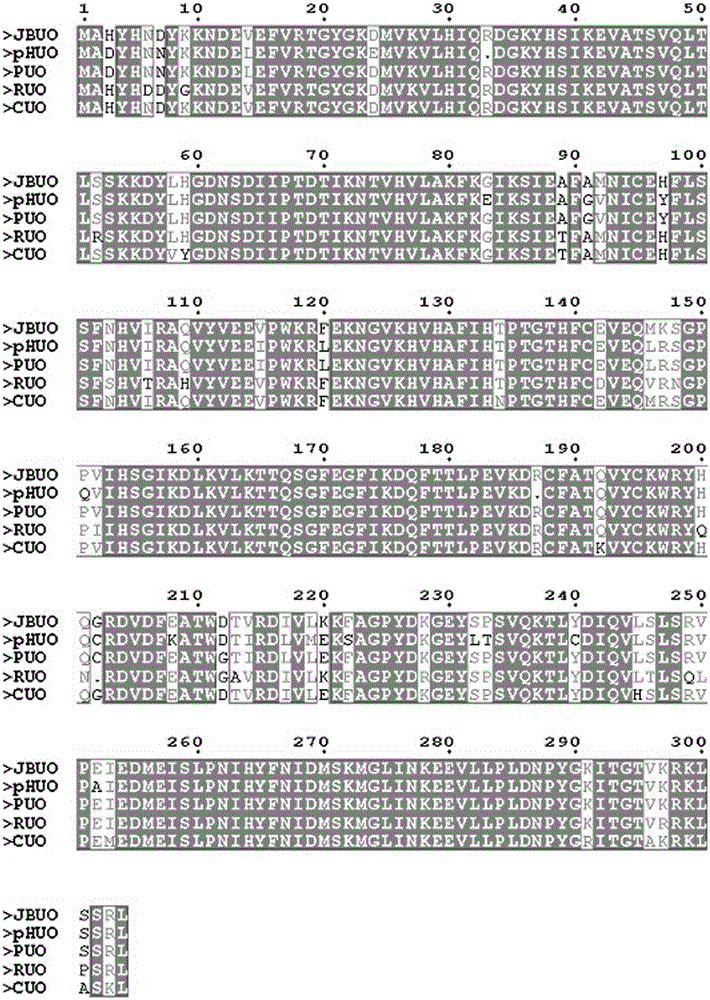
[0032] A method for recombinant expression of recombinant human / baboon chimeric uricase protein JBUO, comprising the steps of:
[0033] (1) Design primers according to the optimized nucleotide sequence of JBUO (Sequence List 1), which need to include enzyme cutting sites, arranged from 5`-3` as follows:
[0034] JBP1: GGAATTCCATATGGCCCATTATCACAACGACTATAAGAA
[0035] JBP2: CCGCTCGAGttaCAGGCGGCTACTCAGTTTGCGT
[0036] Primers were diluted to 50 pmol / [mu]l prior to the PCR step.
[0037] (2) Use Tag enzyme to carry out the PCR step, agarose gel electrophoresis to detect that the JBUO band is located at about 1000bp, recover the target gene from the gel, use NdeI and XhoI to cut the gene enzyme into the sticky end, treat the pET-26b vector in the same way, and add T4DNA Ligase, ligated for 12 hours at 4°C, and transformed into Escherichia coli DH5α. Use the kanamycin sulfate-LB plate to screen and pick out the white spot clone, use the alkaline lysis method or boiling method of ...
Embodiment 2
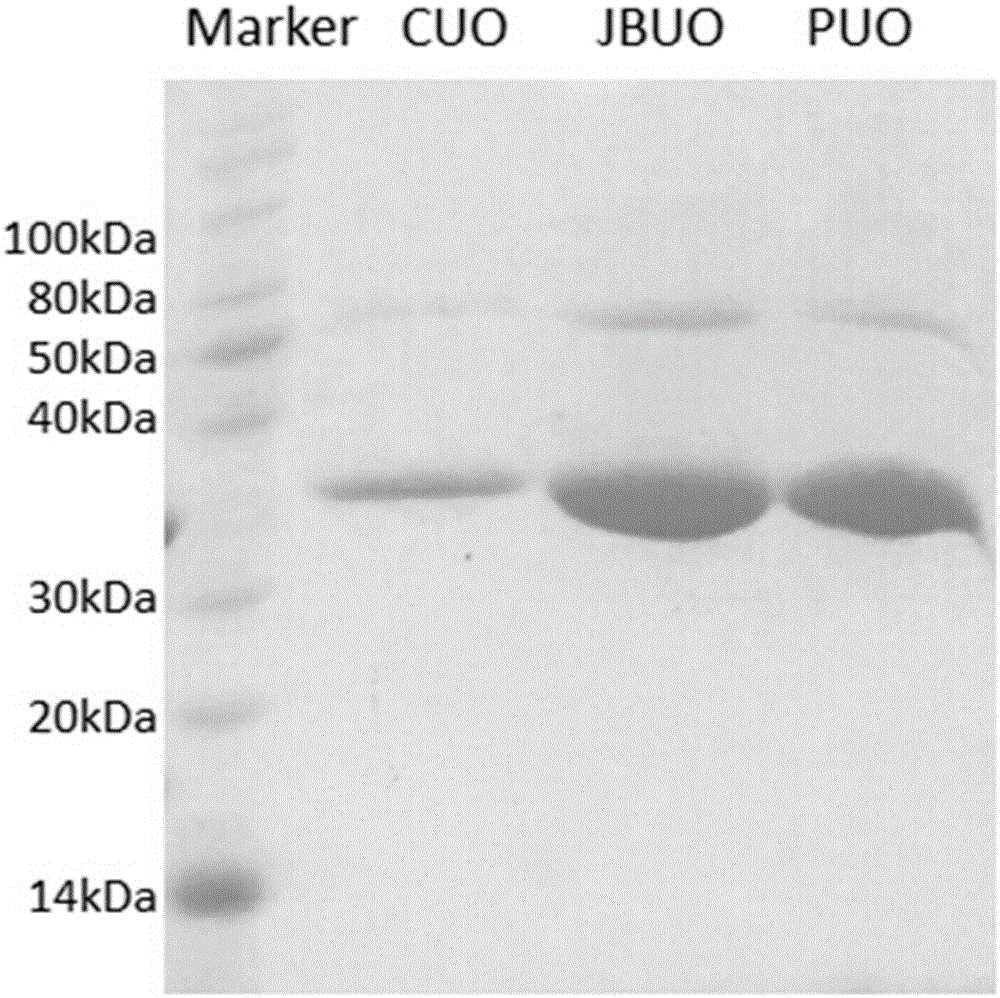
[0042] A method for purifying recombinant human / baboon chimeric uricase protein JBUO, comprising the steps of:
[0043] (1) Choose an anion exchange column with a suitable capacity, and use 0.1M Na with pH 11 2 CO 3 The buffer solution balances the column material, and the solution containing the JBUO protein prepared in Example 1 is slowly loaded onto the column.
[0044] (2), use 0.1M NaHCO with pH8.5 3 Elute the impurity protein with the buffer, wash at least 5 times the volume of the column; then gradually increase the NaCl salt concentration of the buffer to 1.5M, and wash another 5 times the volume of the column to fully elute other impurities.
[0045] (3), use pH11 Na 2 CO 3 The buffer solution flows through the column, and the NaCl salt concentration of the buffer is gradually increased to 1M, and the eluted JBUO protein is collected.
[0046] (4) The molecular sieve on the purified JBUO protein was used to identify the aggregated form, and the peak position was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com