NN ligand, NN ligand iron complex, crystals, preparation method and applications thereof
A technology of iron complexes and ligands, applied in the preparation of carbon-based compounds, the preparation of organic compounds, iron-organic compounds, etc., can solve the problems of low reaction yield, high production cost, poor stereoselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Embodiment 1: prepare NN ligand 6 of the present invention
[0157]
[0158] Add 2-acetyl-6-methylpyridine (2.5g, 18.5mmol, 1.0equiv), 2,6-diisopropylaniline (2.5g, 15.3mmol, 0.77equiv) and p-toluene to a 100ml single-necked bottle successively Sulfonic acid (141.0mg, 741μmol, 4mol%), toluene (30mL), then reflux for 48h and remove the water formed by the reaction through a water separator. After the reaction, the solvent was removed under reduced pressure, the sample was dry-loaded, and separated by column chromatography (v / v, ethyl acetate:n-hexane=1:100) to obtain yellow oil 6a, 3.6g, with a yield of 81%.
[0159] 1 HNMR (400MHz, CDCl 3 )δ=8.16-8.14(d, J=7.6Hz, 1H, Ar-H), 7.70-7.66(t, J=7.6Hz, 1H, Ar-H), 7.24-7.22(m, 1H, Ar-H ),7.16-7.15(m,2H,Ar-H),7.10-7.06(m,1H,Ar-H),2.79-2.72(m,2H,Ar(CH(CH 3 ) 2 ) 2 ),2.619(s,3H,ArCH 3 ),2.21(s,3H,NCCH 3 ), 1.15-1.13(d, J=7.2, 12H, Ar(CH(CH 3 ) 2 ) 2 ). 13 CNMR (101MHz, CDCl 3 )δ=167.50, 157.49, 156.06, 146.75, 136....
Embodiment 2
[0161] Embodiment 2: prepare the NN ligand 6 of the present invention
[0162]
[0163] Add 2-acetylpyridine (3.6g, 30.0mmol, 1.0equiv), 2,6-diethylaniline (3.7g, 25mmol, 0.83equiv), p-toluenesulfonic acid (285.0mg, 1.5 mmol, 5mol%), toluene (40mL), then reflux for 48h and remove the water generated by the reaction through a water separator. After the reaction was completed, the solvent was removed under reduced pressure, the sample was dry-loaded, and column chromatography (v / v, ethyl acetate:n-hexane=1:100) was obtained to obtain NN ligand 6e5.5g in a yellow oil with a yield of 88% .
[0164] 1 HNMR (400MHz, CDCl 3 )δ=8.68-8.66(m,1H,Ar-H),8.38-8.36(m,1H,Ar-H),7.82-7.78(td,J=8.0Hz,1.6Hz,1H,Ar-H), 7.39-7.36(m,1H,Ar-H),7.12-7.10(d,J=7.6Hz,2H,Ar-H),7.05-7.01(m,1H,Ar-H),2.46-2.29(m, 4H,Ar(CH 2 CH 3 ) 2 ),2.02(s,3H,NCCH 3 ), 1.15-1.12(t, J=7.6Hz, 6H, Ar(CH 2 CH 3 ) 2 ). 13 CNMR (101MHz, CDCl 3 )δ=167.01, 156.54, 148.68, 147.84, 136.57, 131.26, 126.02, 124.92, 123....
Embodiment 3
[0177] Embodiment 3: prepare NN ligand iron complex 7 of the present invention
[0178]
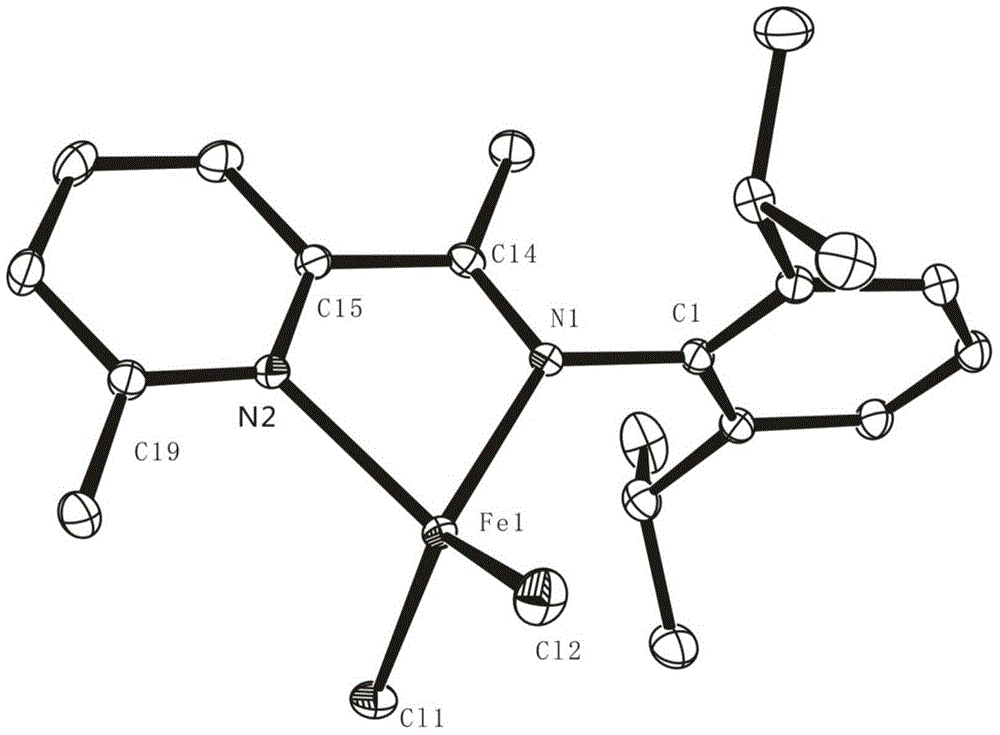
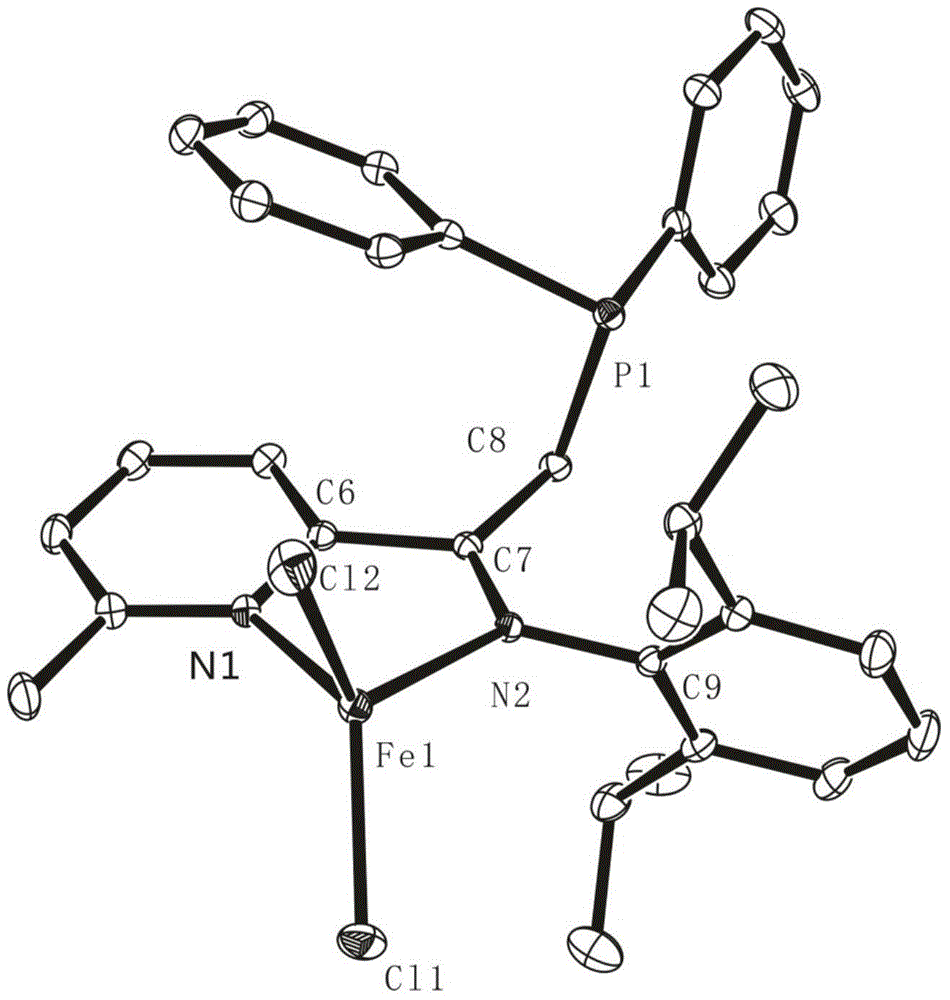
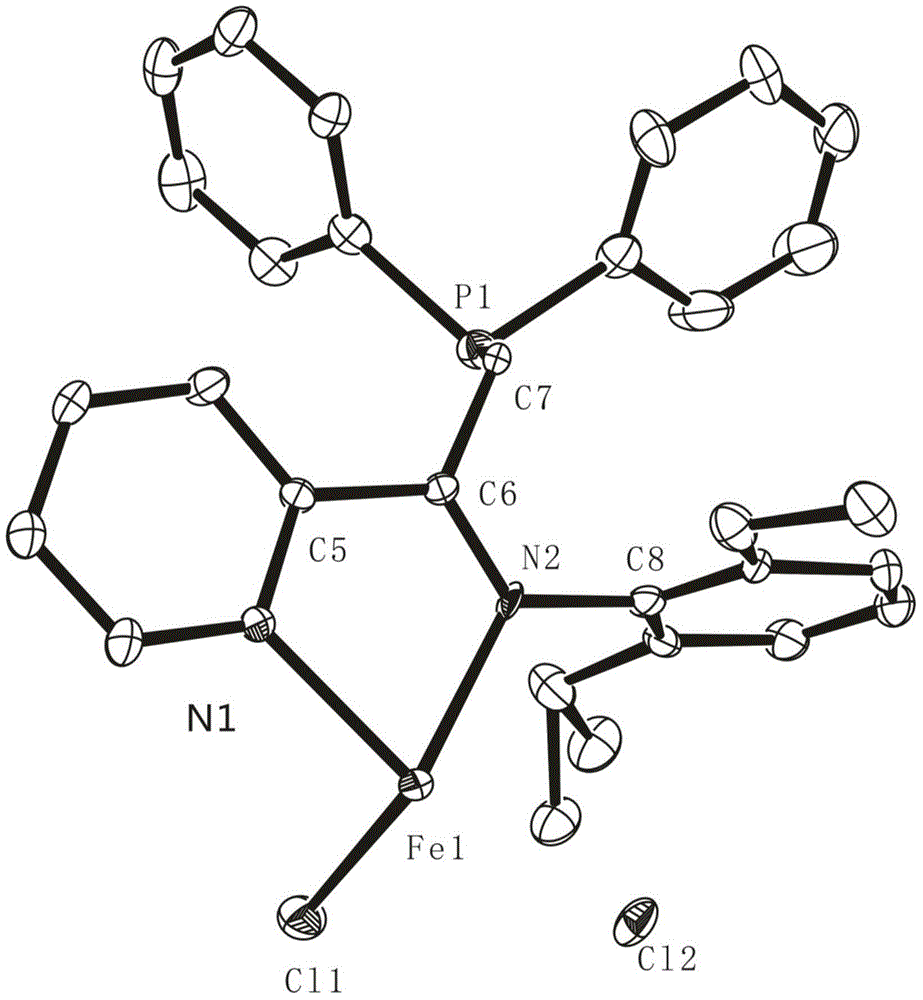
[0179] In a nitrogen glove box, the FeCl 2 (133mg, 1.0mmol, 1.0equiv) and THF (35mL) were added to a 50mL round bottom Schlenk bottle, and the FeCl 2 After complete dissolution, a THF solution (10 mL) of 6a ligand (324 mg, 1.1 mmol, 1.05 equiv) was added dropwise to the above solution, the color gradually turned blue-brown, and the reaction was stirred at room temperature for 24 h. After the reaction is completed, filter with dried diatomaceous earth in the glove box. After the filtrate is concentrated to 1-2 mL, add about 10 mL of n-hexane to it, and a large amount of red solid will be precipitated; then filter the obtained solid with a fine sand core And washed with n-hexane, the solvent was removed under reduced pressure to obtain blue powder 7j. Complex 7j is dissolved in CH 2 Cl 2 / n-hexane mixed solvent (volume ratio 1:7), slowly volatilized to obtain a single crystal, and it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com