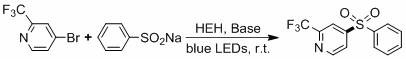Method for preparing heterocyclic sulfone organic compounds
A technology of organic compounds and sulfones, applied in the field of catalytic chemistry, can solve problems such as high reduction potential of aryl halogens, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
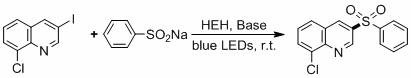
[0023] Example 1: The HEH catalytic system catalyzes the reaction of 4-iodopyridine and sodium benzenesulfinate.
[0024]
[0025] 4-iodopyridine (0.2 mmol), sodium benzenesulfinate (0.4 mmol), Cs 2 CO 3 (0.3 mmol), HEH (20mol%, 0.04 mmol) and DMSO (1 mL) were added to a dry reaction tube with a magnetic stirrer, and then the reaction tube was washed with N 2 Replaced 3 times, stirred and reacted for 24 h under the irradiation of blue LED. After the reaction, 5 mL of water was added, then extracted with 3×5 mL of ethyl acetate, the organic phases were combined, and the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated by rotary evaporation and separated by silica gel column chromatography. The target product was obtained (68% yield). will be replaced by K 2 CO 3 , and the rest remained unchanged, the target product was obtained (yield 62%).
[0026] 1 H-NMR (400 MHz, CDCl 3 , ppm): δ 8.80 (s, 2H), 7.95 (d, J = 7.1 H...
Embodiment 2
[0028] Example 2: The HEH catalytic system catalyzes the reaction of 4-bromo-2-trifluoromethylpyridine and sodium benzenesulfinate.
[0029]
[0030] 4-Bromo-2-trifluoromethylpyridine (0.2 mmol), sodium benzenesulfinate (0.4 mmol), Cs 2 CO 3 (0.3 mmol), HEH (20 mol%) and DMSO (1 mL) were added to a dry reaction tube with a magnetic stirrer, and then the reaction tube was washed with N 2 Replaced 3 times, stirred and reacted for 24 h under the irradiation of blue LED. After the reaction, 5 mL of water was added, then extracted with 3×5 mL of ethyl acetate, the organic phases were combined, and the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated by rotary evaporation and separated by silica gel column chromatography. The target product was obtained (yield 85%).
[0031] 1 H NMR (400 MHz, CDCl 3 , ppm): δ = 8.94 (d, J = 4.9 Hz, 1H), 8.13 (s,1H), 7.98 (t, J = 7.3 Hz, 3H), 7.69 (t, J = 7.4 Hz, 1H), 7.60 (t, J = 7.6 ...
Embodiment 3
[0033] Example 3: The HEH catalytic system catalyzes the reaction of 2-bromo-6-trifluoromethylpyridine and sodium benzenesulfinate.
[0034]
[0035] 2-Bromo-6-trifluoromethylpyridine (0.2 mmol), sodium benzenesulfinate (0.4 mmol), Cs 2 CO 3(0.3 mmol), HEH (20 mol%) and DMSO (1 mL) were added to a dry reaction tube with a magnetic stirrer, and then the reaction tube was washed with N 2 Replaced 3 times, stirred and reacted for 24 h under the irradiation of blue LED. After the reaction, 5 mL of water was added, then extracted with 3×5 mL of ethyl acetate, the organic phases were combined, and the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated by rotary evaporation and separated by silica gel column chromatography. The target product was obtained (yield 81%).
[0036] 1 H NMR (400 MHz, CDCl 3 , ppm): δ = 8.38 (d, J = 7.9 Hz, 1H), 8.12 (dd, J = 14.1, 7.6 Hz, 3H), 7.82 (d, J = 7.8 Hz, 1H), 7.65 (t, J = 7.3 Hz, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com