17-hydroxycorticosteroid immunodetection reagent and preparation method thereof
A technology of hydroxycorticosteroids and reagents, applied in the biological field, can solve problems such as unsuitable for large-scale use, backward technology, poor stability, etc., and achieve the effects of being beneficial to clinical promotion and use, reducing detection costs, and strong binding force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 7
[0059] Synthesis of embodiment one 17-hydroxycorticosteroid immunogen
[0060] The 17-hydroxycorticosteroid immunogen is composed of bovine serum albumin (BovineSerumAlbumin, BSA) and 17-hydroxycorticosteroid derivatives represented by formula (II) The specific steps are as follows:
[0061] 1. Dissolve 200mg of bovine serum albumin in 50ml of 0.2M, pH8.5 phosphate buffer;
[0062] 2. Add the following chemicals into a small beaker and stir to dissolve: 200mg of 17-hydroxycorticosteroid derivatives, 3.5ml of dimethylformamide, 3.5ml of ethanol, 7.0ml of 10mM potassium phosphate buffer at pH 5.0, 200mg of 1-ethyl -3-(-3-dimethylaminopropyl) carbodiimide, 50mg N-hydroxyl sulfosuccinimide, these chemicals were stirred and dissolved at room temperature for 30 minutes;
[0063] 3. Add the dissolved solution to the BSA solution dropwise, and stir overnight at 2-8°C to obtain the antigen; purify the synthesized antigen by dialysis to obtain the 17-hydroxycorticosteroid immunogen...
Embodiment 2
[0064] Example 2: Preparation of Anti-17-Hydroxycorticosteroid Specific Antibody
[0065] The 17-hydroxycorticosteroid immunogen prepared in Example 1 was inoculated into experimental animal rabbits by conventional methods, and the antiserum was taken after booster immunization. The specific steps were as follows:
[0066] 1. Dilute the above-mentioned synthetic 17-hydroxycorticosteroid immunogen to 1.0 mg / ml with PBS to obtain an antigen solution, then mix 1.0 ml of the antigen solution with an equal amount of Freund's complete adjuvant, and inject the experimental animal rabbit.
[0067] 2. After 2-3 weeks, inject 1.0ml of the same antigen solution and the same amount of Freund's incomplete adjuvant to the above-mentioned experimental animal rabbit once, and then inject once every four weeks, a total of 4 injections.
[0068] 3. Take blood from the experimental animal rabbit in step 2, separate and purify to obtain anti-17-hydroxycorticosteroid specific antibody with a tit...
Embodiment 3
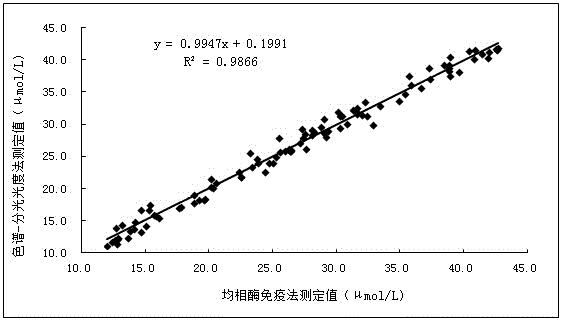
[0069] Embodiment three: the ELISA test of 17-hydroxycorticosteroid
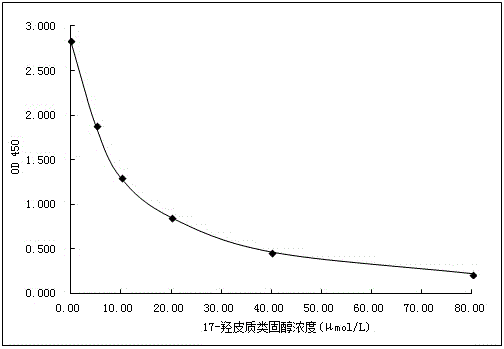
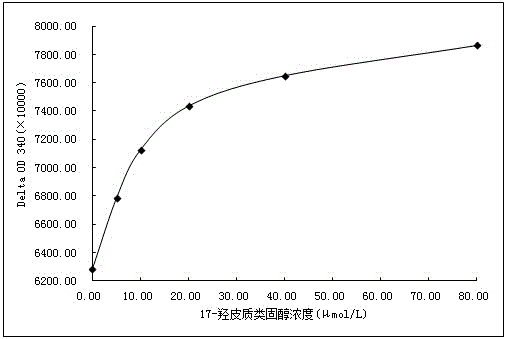
[0070] 1.17-Hydroxycorticosteroid ELISA detection standard curve establishment
[0071] (1) Preparation of standard products
[0072] 17-Hydroxycorticosteroid powder (purchased from Sigma Company) was dissolved in methanol solution to prepare a 1000 μmol / L stock solution. The stock solution was sequentially diluted with ELISA buffer to 80.00 μmol / L, 40.00 μmol / L, 20.00 μmol / L, 10.00 μmol / L, 5.00 μmol / L and 0.00 μmol / L standard solutions. Among them, the ELISA buffer contains 50.0mM Tris, 145mM NaCl and 0.25% BSA.
[0073] (2) Utilize the ELISA test method of 17-hydroxycorticosteroid to prepare standard curve
[0074] Dilute the anti-17-hydroxycorticosteroid-specific antibody prepared in Example 2 with PBS to a final concentration solution of 1:8000, coat 100 μL / well on a 96-well enzyme-linked plate, and place it at 4°C for 12-24h; After washing the 96-well enzyme-linked plate coated with anti-17-hydrox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com