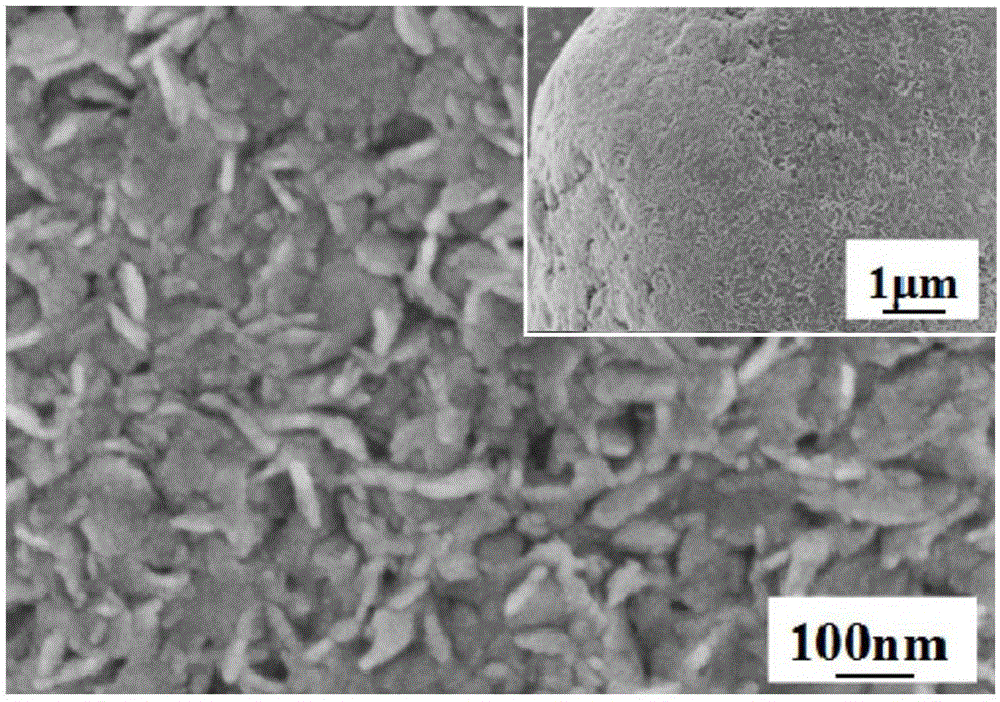
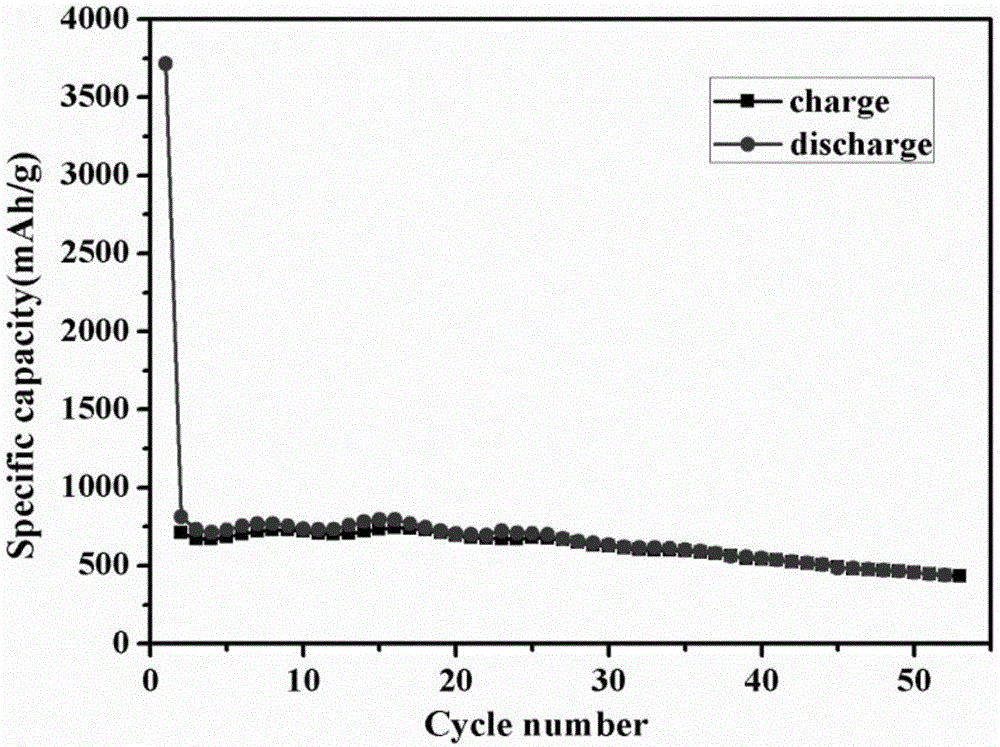
Preparation method for nano-flake SnS2 sodium-ion battery negative electrode material with good rate capability
A sodium-ion battery, nano-sheet technology, applied in battery electrodes, active material electrodes, negative electrodes, etc., can solve the problems of irregular particle morphology and size, poor product quality, incomplete reaction, etc. The effect of uniform appearance, fast heating speed and full and thorough response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0031] 1) SnCl 4 ·5H2 O was dissolved in deionized water to prepare solution A with a concentration of 0.5 mol / L, and at the same time, 1 mol / L of HCl or NH 4 ·H 2 O adjusts the pH of solution A to 1, NaS·9H 2 O was dissolved in deionized water to prepare solution B with a concentration of 0.1 mol / L.
[0032] 2) Dilute the two solutions of A and B according to the element molar ratio n Sn :n S = 1.0:2.0 was mixed to obtain solution C, and magnetically stirred at 26°C for 10 minutes to form a uniform and stable mixed solution D.
[0033] 3) Put the D solution into a homogeneous hydrothermal reaction kettle, seal the hydrothermal kettle, control the volume filling ratio to 50%, control the reaction temperature at 120°C, control the reaction time at 8h, and then turn off the power.
[0034] 4) After the reaction kettle was cooled, the precursor was taken out, separated by centrifugal washing, washed three times with deionized water, and then washed three times with absolute ...
specific Embodiment 2
[0035] 1) SnCl 4 ·5H 2 O was dissolved in deionized water to prepare a solution A with a concentration of 1mol / L, and at the same time, 1mol / L HCl or NH 4 ·H 2 O adjusts the pH of solution A to 7, NaS·9H 2 O was dissolved in deionized water to prepare solution B with a concentration of 2 mol / L.
[0036] 2) Dilute the two solutions of A and B according to the element molar ratio n Sn :n S =2:3 ratio mixed to obtain solution C, magnetically stirred at 26°C for 5min to form a uniform and stable mixed solution D.
[0037] 3) Put the D solution into a homogeneous hydrothermal reaction kettle, seal the hydrothermal kettle, control the volume filling ratio to 50%, control the reaction temperature at 160°C, control the reaction time at 12h, and then turn off the power.
[0038] 4) After the reaction kettle was cooled, the precursor was taken out, separated by centrifugal washing, washed twice with deionized water, and then washed twice with absolute ethanol to obtain a yellow-br...
specific Embodiment 3
[0039] 1) SnCl 4 ·5H 2 O was dissolved in deionized water to prepare a solution A with a concentration of 1.2 mol / L, and at the same time, 1 mol / L HCl or NH 4 ·H 2 O adjusts the pH of solution A to 9, NaS·9H 2 O was dissolved in deionized water to prepare solution B with a concentration of 2.4mol / L.
[0040] 2) Dilute the two solutions of A and B according to the element molar ratio n Sn :n S =2.5:4.3 ratio mixed to obtain solution C, magnetically stirred at 26°C for 30min to form a uniform and stable mixed solution D.
[0041] 3) Put the D solution into a homogeneous hydrothermal reaction kettle, seal the hydrothermal kettle, control the volume filling ratio to 60%, control the reaction temperature at 200°C, control the reaction time at 16h, and then turn off the power.
[0042] 4) After the reaction kettle was cooled, the precursor was taken out, separated by centrifugal washing, washed three times with deionized water, and then washed three times with absolute ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com