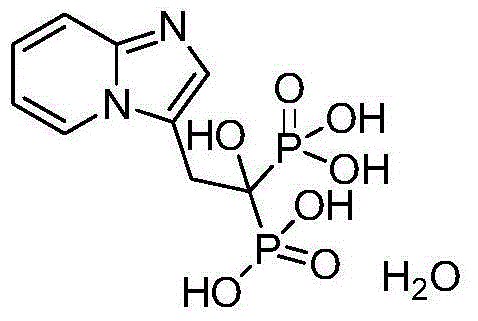
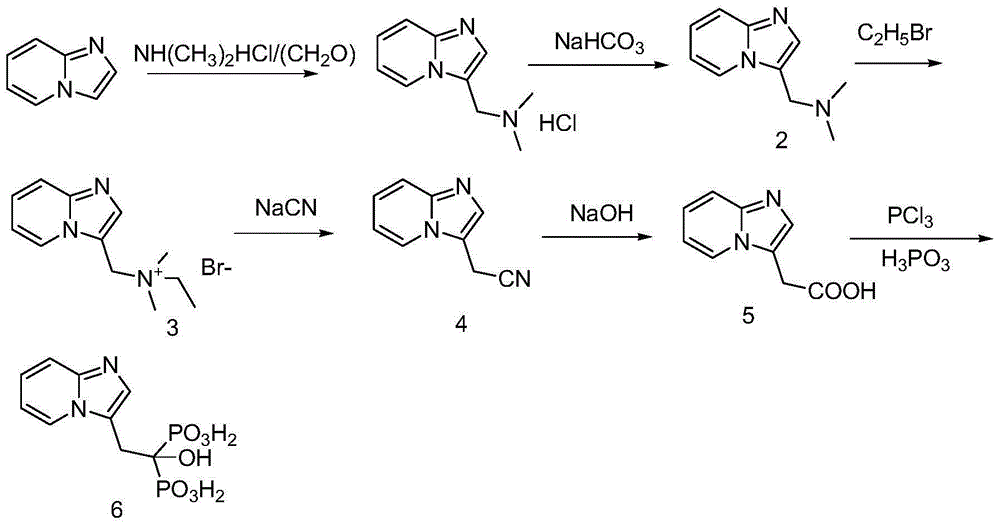
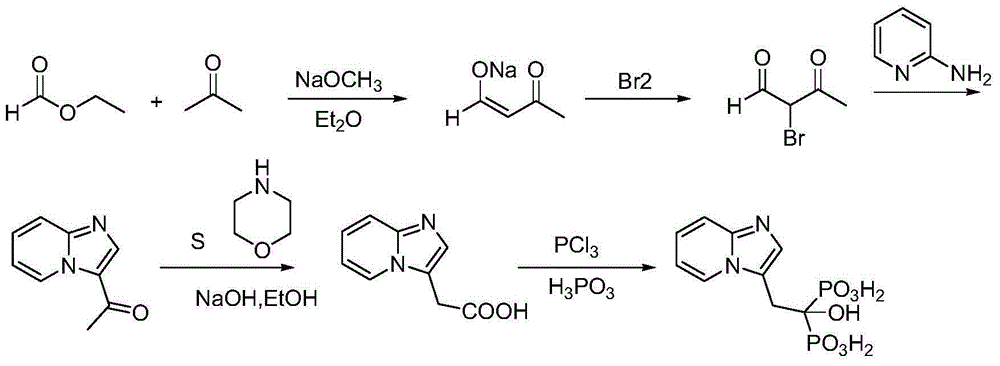
Method for producing minodronic acid monohydrate by using water as solvent
A minodronic acid monohydrate and solvent technology, which is applied in the field of minodronic acid hydrate production, can solve the problems of inconvenient production process and increased cost, achieve significant economic value, low production cost, and improve the effect of drug purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Step a: Preparation of ethyl 2-[imidazo(1,2-a)pyridin-3-yl]acetate (B)
[0076]
[0077] Weigh A: 9.4g or 0.1mol of 2-aminopyridine, 20.9g or 0.1mol of ethyl 4-bromoacetoacetate and 1.1g or 0.01mol of DABCO, and dissolve them in 400mL of water. Heat to 60-70°C, monitor the reaction by TLC, and the reaction is complete in about 1 hour. Extracted three times with 200 mL of ethyl acetate, combined the organic layers, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain a crude product. The crude product was recrystallized from a mixture of 100 mL of petroleum ether and ethyl acetate, wherein the volume ratio of petroleum ether to ethyl acetate was 1:3 to obtain 18 g of light yellow solid with a yield of 88.1%.
[0078] ESI-MS:[M+H] + =205.1
[0079] NMR data: 1 H-NMR (CDCl 3 ,400MHz): δ(ppm)=8.06(d,J=6.8Hz,1H),7.62(d,J=8.8Hz,1H),7.56(s,1H),7.20(t,1H),4.14-4.20 (dd, J=7.2Hz, 2H), 3.94(s, ...
Embodiment 2
[0090] Step a: Preparation of methyl 2-[imidazo(1,2-a)pyridin-3-yl]acetate (B)
[0091]
[0092]Weigh A: 128g of 2-aminopyridine, namely 1.36mol, 318g of methyl 4-bromoacetoacetate, namely 1.63mol, and 30.5g of DABCO, namely 0.272mol, and dissolve them in 2.5L of water. Heating to 60-70°C, monitoring the reaction by TLC, the reaction was complete in about 1.5 hours. Extracted 3 times with 2 L of ethyl acetate, combined the organic layers, washed with 750 mL of saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain a crude product. The crude product was recrystallized from a mixture of 470 mL of petroleum ether and ethyl acetate, wherein the volume ratio of petroleum ether to ethyl acetate was 2:5, to obtain 135 g of a light yellow solid with a yield of 52.2%.
[0093] ESI-MS:[M+H] + =191.1
[0094] NMR data: 1 H-NMR (CDCl 3 ,400MHz): δ(ppm)=8.06(d,J=6.8Hz,1H),7.62(d,J=8.8Hz,1H),7.56(s,1H),7.20(t,1H),4.25(s...
Embodiment 3
[0103] Step a: Preparation of ethyl 2-[imidazo(1,2-a)pyridin-3-yl]acetate (B)
[0104]
[0105] Weigh A: 18.8g of 2-aminopyridine, namely 0.2mol, 42.9g of ethyl 4-bromoacetoacetate, namely 0.22mol, and 20.2g of triethylamine, namely 0.2mol, and dissolve them in 380mL of water. Heating to 60-70°C, monitoring the reaction by TLC, the reaction was complete in about 5 hours. Extracted three times with 150 mL of ethyl acetate, combined the organic layers, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain a crude product. The crude product was recrystallized from a mixture of 100 mL of petroleum ether and ethyl acetate, wherein the volume ratio of petroleum ether to ethyl acetate was 1:3, to obtain 25.2 g of a light yellow solid with a yield of 61.2%.
[0106] Step b: Preparation of 2-[imidazo(1,2-a)pyridin-3-yl]ethyl acetate (C)
[0107]
[0108] Dissolve B: 75 g of ethyl 2-(imidazo[1,2-a]pyrid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com