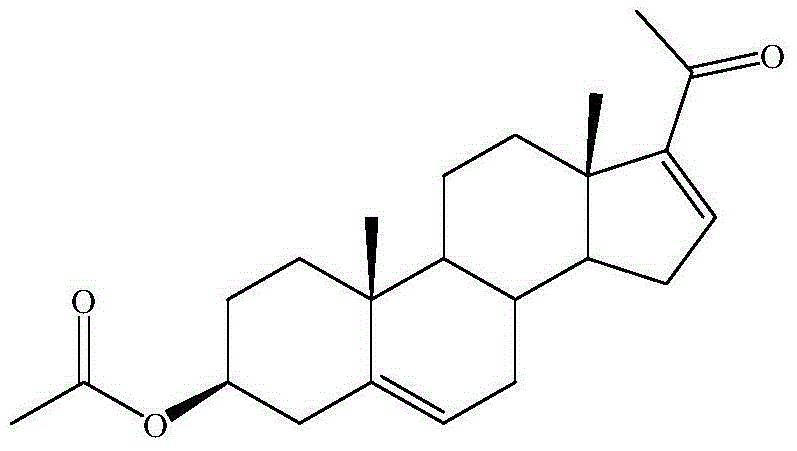
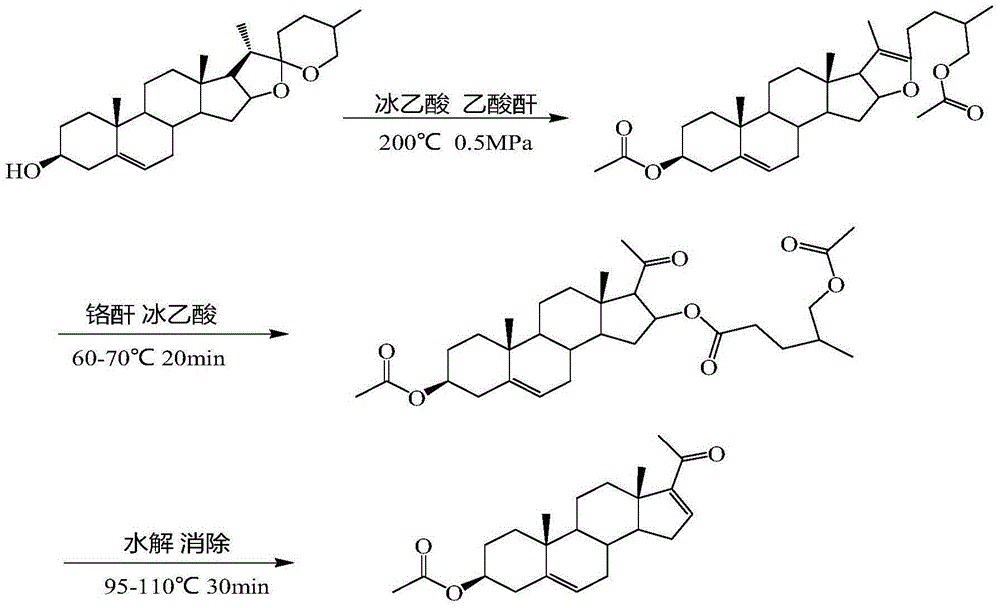
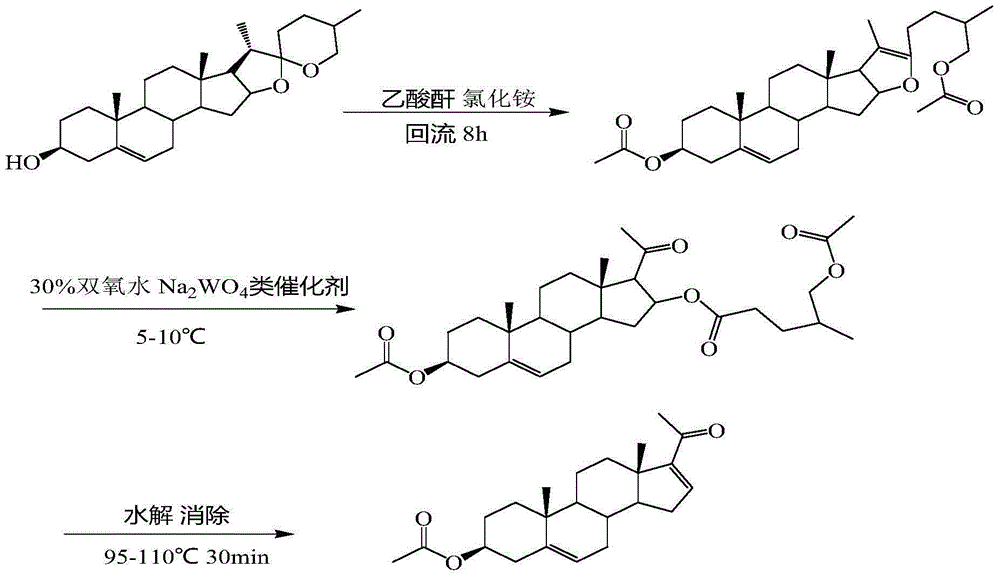
Preparation method of dehydropregnenolone acetate
A technology of dienolone acetate and acetic anhydride, which is applied in the direction of steroids and organic chemistry, can solve the problems of oxidant improvement, experiment and production site limitations, and the problem of chromium compound pollution has not been completely solved, and the product yield can be achieved Stable, low cost, less pollution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A thermometer and a reflux condenser were sequentially installed on a 250ml three-necked flask, and 10g (24.1mmol) of diosgenin, 1.42g (26.5mmol) of ammonium chloride, 150ml of dry acetic anhydride, 0.5ml of pyridine, and a magnetic stirrer were added successively. After plugging with a glass stopper, heat to 90° C. on a collector-type constant temperature heating magnetic stirrer (oil bath) to see that the diosgenin is completely dissolved, and continue to heat up to 140° C. and reflux for 8 hours to stop the reaction. Take the reaction bottle out of the oil bath, cool it down to room temperature naturally and add 40ml of glacial acetic acid, put the reaction bottle into a low-temperature reactor to cool down to about 5°C, slowly drop in the prepared oxidizing solution, keep it warm for 2 hours, and then raise the temperature naturally After reaching room temperature, carry out hydrolysis again, move the reaction bottle into an oil bath, raise the temperature to 100°C, ...
Embodiment 2
[0036] Whole reaction procedure is identical with example 1, and the amount of ammonium chloride in the change experiment process is 1.23g (23.0mmol), and final gained product crude product 6.4g, obtains product 2.12g after recrystallization. The purity is 96.5%, and the yield is 24.7%.
Embodiment 3
[0038] Whole reaction procedure is identical with example 1, and the amount of ammonium chloride in the change experiment process is 1.55g (28.9mmol), and the final gained product crude product 6.8g, obtains product 2.82g after recrystallization. The purity is 94.4%, and the yield is 32.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com