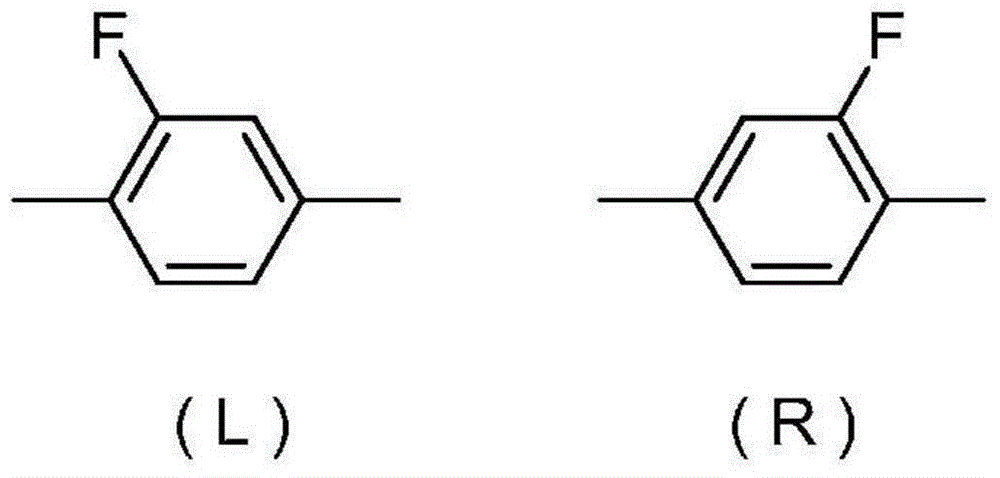
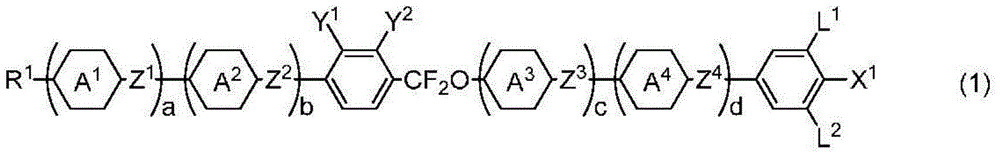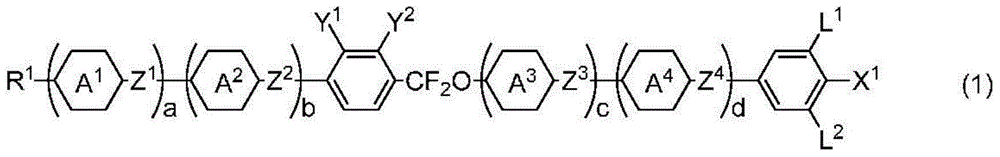Liquid-crystalline compound having difluoromethyleneoxy group, liquid crystal composition, and liquid crystal display element
一种液晶组合物、化合物的技术,应用在有机化学、液晶材料、非线性光学等方向,能够解决介电常数不足够大等问题,达到透明点高、短响应时间、低阈电压的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0247] Composition (1) is prepared by a method such as dissolving required components at high temperature. Depending on the application, additives may be added to the composition. Examples of additives are optically active compounds, polymerizable compounds, polymerization initiators, antioxidants, ultraviolet absorbers, light stabilizers, heat stabilizers, defoamers, pigments, and the like. Such additives are well known to those skilled in the art and are described in the literature.
[0248] Composition (1) may further contain at least one optically active compound. The optically active compound has an effect of preventing reverse twist by imparting a desired torsion angle (torsion angle) by generating a helical structure in the liquid crystal molecule. Preferable examples of the optically active compound include the following compound (Op-1) to compound (Op-18).
[0249]
[0250] In compound (Op-18), ring F is 1,4-cyclohexylene or 1,4-phenylene, R 21 is an alkyl grou...
Embodiment 1
[0306] Synthesis of compound (No.1-2-2)
[0307]
[0308] Step 1
[0309] Under nitrogen atmosphere, 2,2,6,6-tetramethylpiperidine (48.5ml) and tetrahydrofuran (THF) (250ml) were added into the reactor, and cooled to 0°C. Thereto, n-butyllithium (1.60 M, n-hexane solution, 178 ml) was slowly added, followed by stirring for 1 hour. Then cool to -70°C, slowly add compound (T-1) (50.0g) in THF (500ml) solution, after stirring for 1 hour, slowly add DMF (N,N-dimethylformylfluorene) (40.1ml) , and stirred for 12 hours while returning to room temperature. The reaction mixture was poured into saturated aqueous ammonium chloride, and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine and dried over anhydrous magnesium sulfate. The solution was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (volume ratio, hexane:ethyl acetate=5:1). Furthermore, it purified by recrystallization in...
Embodiment 2
[0323] Synthesis of compound (No.1-4-21)
[0324]
[0325] Step 1
[0326] Under nitrogen atmosphere, compound (T-8) (50.0 g) and diethyl ether (800 ml) were added into the reactor, and cooled to -70°C. Thereto, n-butyllithium (1.63 M, n-hexane solution, 112 ml) was slowly added, followed by stirring for 2 hours. Then, a diethyl ether (50.0 ml) solution of trimethyl borate (22.8 g) was slowly added, and the mixture was stirred for 12 hours while returning to room temperature. Then, it was cooled to -30°C, 6N hydrochloric acid (150 ml) was slowly added, and the mixture was stirred for 3 hours while returning to room temperature. The reaction mixture was poured into water, and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine and dried over anhydrous magnesium sulfate. The solution was concentrated under reduced pressure, and purified by recrystallization from heptane to obtain compound (T-9) (29.2 g, 78%).
[0327] St...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com