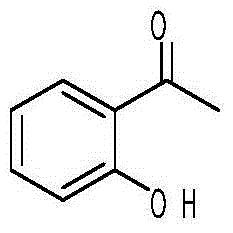
Preparation method of 2-hydroxyacetophenone
A technology of o-hydroxyacetophenone and phenol, applied in the field of preparation of o-hydroxyacetophenone and o-hydroxyacetophenone, can solve the problems of low yield, complicated preparation method and process, and achieves high yield, low cost, The effect of low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
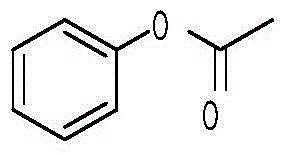
[0032] 1) Preparation of phenol acetate
[0033] Add 14.1g (0.15mol) of phenol, 14.13g (0.18mol) of acetyl chloride, and 40ml of cyclohexane into a three-neck flask, and react at room temperature for 2-3 hours. After the plate reaction is completed, adjust the pH to about 8 with sodium bicarbonate solution. The organic phase was collected and dried over anhydrous sodium sulfate to obtain 20.2 g of the product light yellow oily liquid phenol acetate (yield 99%).
[0034] 2) the preparation of o-hydroxyacetophenone
[0035] a. Add 13.6g (0.1mol) of phenol acetate in a three-necked flask, add 16g (0.12mol) of aluminum trichloride, heat and reflux at 120°C for 1.5h, after the plate reaction is completed, add 50ml of mass fraction of 5% hydrochloric acid solution, extracted 3 times with ethyl acetate, collected the organic phase, extracted with the second organic solvent; concentrated the organic layer, added 1-2 times the volume of the third organic solvent, the organic layer was...
Embodiment 2
[0040] 1) Preparation of phenol acetate
[0041] Add 14.1g (0.15mol) of phenol, 14.13g (0.18mol) of acetyl chloride, and 240ml of chloroform into a three-neck flask, and react at room temperature for 2-3 hours. After the plate reaction is completed, adjust the pH to about 8 with sodium bicarbonate solution. Collect the organic phase and dry it over anhydrous sodium sulfate to obtain 12.05 g of product light yellow oily liquid phenol acetate (yield 59%)
[0042] 2) the preparation of o-hydroxyacetophenone
[0043] a. Add 13.61g (0.1mol) of phenol acetate to a three-necked flask, add 16g (0.12mol) of aluminum trichloride, heat and reflux at 130°C for 1.5h, after the plate reaction is complete, add 50ml of mass fraction to 5 % hydrochloric acid solution, extracted 3 times with ethyl acetate, concentrated the organic layer, added 1-2 times the volume of the third organic solvent, the organic layer was frozen and filtered, and the operation was repeated 2-3 times to obtain the fil...
Embodiment 3
[0047] 1) Preparation of phenol acetate
[0048] Add 14.1g (0.15mol) of phenol, 14.13g (0.18mol) of acetyl chloride, and 40ml of toluene into a three-necked flask, and react at room temperature for 2-3h. After the plate reaction is completed, adjust the pH to about 8 with sodium bicarbonate solution. Collect the organic phase and dry it over anhydrous sodium sulfate to obtain 17.77 g (yield 87%) of product light yellow oily liquid phenol acetate
[0049] 2) the preparation of o-hydroxyacetophenone
[0050] a. Add 13.6 g (0.1 mol) of phenol acetate in a three-necked flask, add 16 g (0.12 mol) of aluminum trichloride, heat and reflux at 140 ° C for 1.5 h, and after the plate reaction is complete, add 50 ml with a mass fraction of 5% hydrochloric acid solution, extract 3 times with ethyl acetate, concentrate the organic layer, add 1-2 times the volume of the third organic solvent, freeze the organic layer, filter, repeat the operation 2-3 times, the obtained filter cake, The pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com