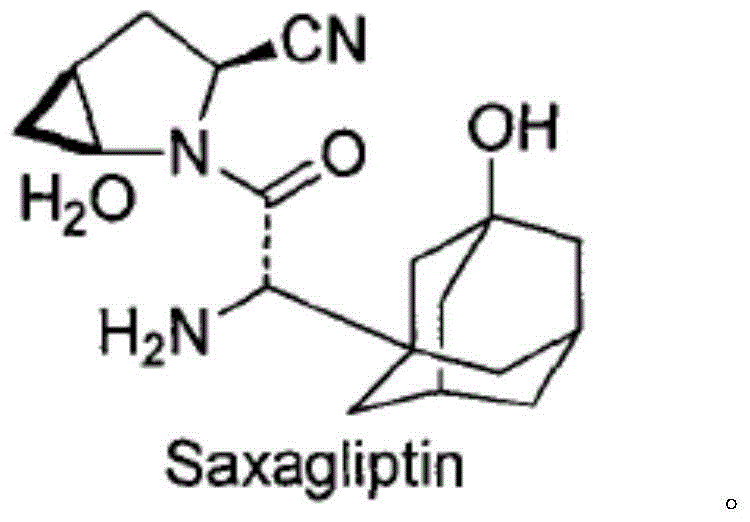
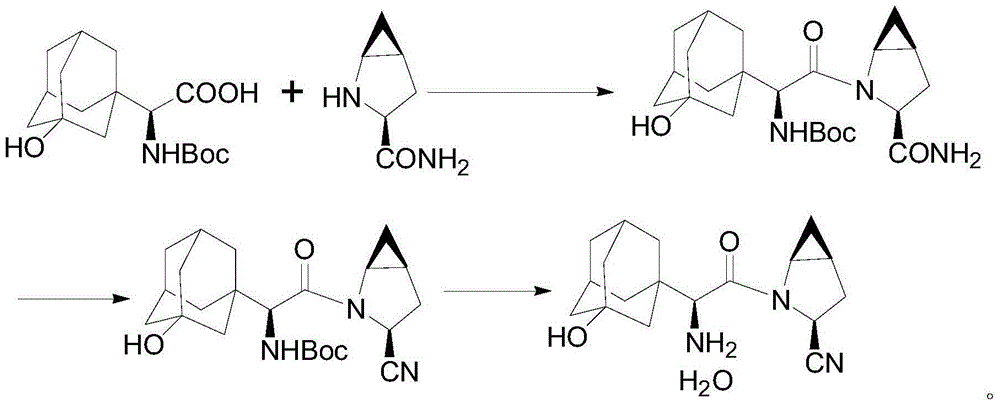
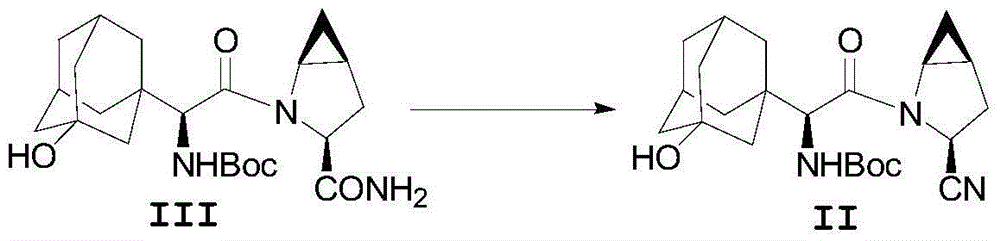
Saxagliptin and saxagliptin salt preparation method
A compound and reaction technology, applied in the field of preparing saxagliptin and its salts, can solve the problems of not being suitable for large-scale industrial production, serious environmental impact, complicated post-treatment, etc., and achieve cheap raw materials, simple post-reaction treatment, and high reaction efficiency. Mild and easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of formula II compound
[0024]
[0025] The compound of formula III (43.3g, 0.1mol) was dissolved in a mixed solvent formed by 100mL N,N-dimethylformamide and 330mL ethyl acetate, then lowered to 0-10°C, and cyanuric chloride (14.8g , 0.08mol), keep warm at 0-10°C and stir for 2-6 hours, add 400mL of water, stir for 2-3 hours, adjust the pH ≈ 8 in the reaction system with sodium hydroxide solution, separate the liquid, and use dichloromethane for the water layer Extract, combine the organic phases, and concentrate under reduced pressure to obtain 34.46 g of the compound of formula II with a molar yield of 83.3% and an HPLC purity of 96.8%.
Embodiment 2
[0026] Embodiment 2: the preparation of formula II compound
[0027] The compound of formula III (43.3g, 0.1mol) was dissolved in a mixed solvent formed by 100mL N,N-dimethylformamide and 330mL dichloromethane, then lowered to 0-10°C, and cyanuric chloride (14.8g, 0.08mol), keep warm at 0-10°C and stir for 2-6 hours, add 400mL water, stir for 2-3 hours, adjust the pH ≈ 8 in the reaction system with sodium hydroxide solution, separate the layers, and extract the water layer with dichloromethane , the organic phases were combined and concentrated under reduced pressure to obtain 35.9 g of the compound of formula II with a molar yield of 86.5% and an HPLC purity of 97.6%.
Embodiment 3
[0028] Embodiment 3: the preparation of formula I compound
[0029]
[0030] Dissolve the compound of formula II (34.5g, 0.1mol) in 100mL of dichloromethane, add hydrochloric acid-diethyl ether solution dropwise at 0-5°C, after dropping, keep warm at 0-5°C and stir for 1-2 hours, then concentrate under reduced pressure The reaction liquid obtained 34.9 g of saxagliptin hydrochloride, and the molar yield was 99.2%.
[0031] Dissolve the above-mentioned saxagliptin hydrochloride in 30 mL of water, add dropwise sodium hydroxide solution at 0-10°C to adjust the pH to ≈8.0, then extract with dichloromethane for 3 times, collect the organic phase, and concentrate under reduced pressure. 30.1 g of saxagliptin was obtained with a molar yield of 96.0% and an HPLC purity of 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com