Quinoline-modified pillararene and preparation thereof and application thereof in performing fluorescence detection on CN<-> in water-containing system
A technology for fluorescence detection and column aromatics, which can be used in fluorescence/phosphorescence, luminescent materials, measuring devices, etc., and can solve problems such as inability to detect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, the preparation of the columnarene modified by quinoline
[0030] (1) Synthesis of intermediates: Take p-hydroxyanisole (2.48g, 20mmol), potassium carbonate (8.4g, 60mmol), potassium iodide (3.3g, 20mmol), 1,4-dibromobutane (17.3g, 80mmol) and acetone (400mL), added to a 500mL round-bottomed flask, refluxed at 60°C for 72h, suction filtered after the reaction, and separated by column chromatography (petroleum ether / ethyl acetate (v / v)=50:1) , to obtain a white solid intermediate (12.8g, yield 65%).
[0031] 1 HNMR (600MHz, CDCl 3 )δ6.83(s,4H),3.94(t, J =6.1Hz,2H),3.83–3.69(m,3H),3.48(t, J =6.7Hz, 2H), 2.11–2.00(m, 2H), 1.97–1.84(m, 2H).
[0032] (2) Synthesis of unilateral column 5 aromatics: take the intermediate (1.32g, 5mmol), p-xylylene dimethyl ether (2.76g, 20mmol), add to 80mL1,2-dichloroethane, stir to dissolve, add Paraformaldehyde (0.75g, 25mmol), boron trifluoride diethyl ether (3.2mL, 25mmol), react at room temperature for 1h; after the r...
Embodiment 2
[0038] Example 2, quinoline-modified columnarene fluorescence monitoring CN -
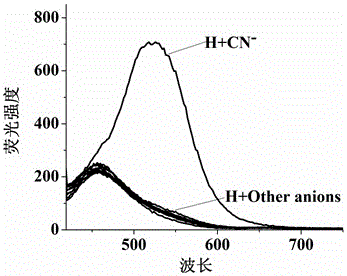
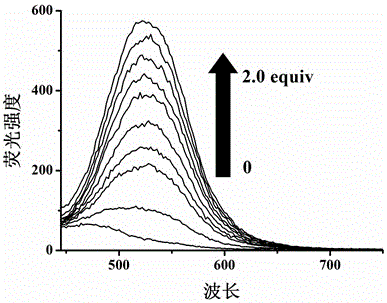
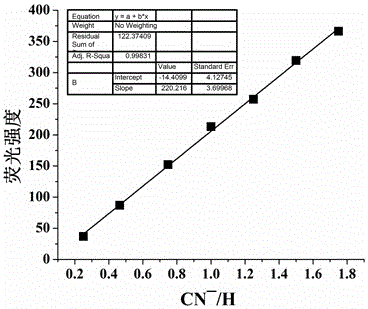
[0039] The quinoline-modified pillararene was configured into an acceptor solution (2×10 -4 mol / L), respectively take 5mL of the receptor solution in a series of 10mL colorimetric tubes, and the solution has no fluorescence. Add F to the colorimetric tube respectively - , Cl - 、Br - , I - 、AcO - 、H 2 PO 4 - 、HSO 4 - , ClO 4 - 、CN - 、SCN - DMSO solution (0.01mol / L) 0.5mL. If the fluorescence of the acceptor solution is turned on and produces a strong yellow-green fluorescence, it means that CN is added. - ; If there is no fluorescence in the acceptor solution (fluorescence cannot be turned on), it means that the addition of CN is not - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com