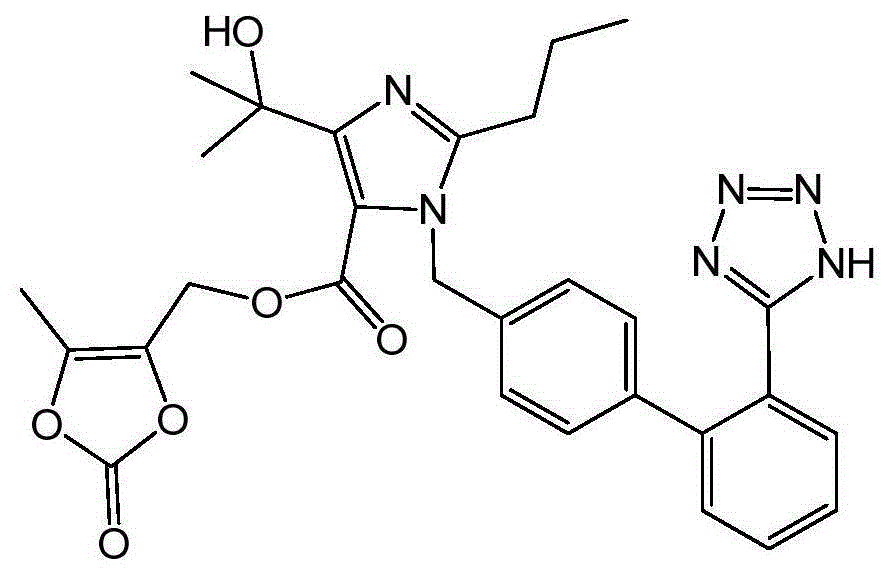
Purification method of olmesartan
A technology for olmesartan medoxomil and a purification method, which is applied in the field of purification of high-purity bulk drug products, can solve the problems of easy excess of residual solvent in the product, difficult to produce by industrialized methods, slow crystallization speed, etc., and achieves low degradation risk and drying. The effect of short time and fast crystallization speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
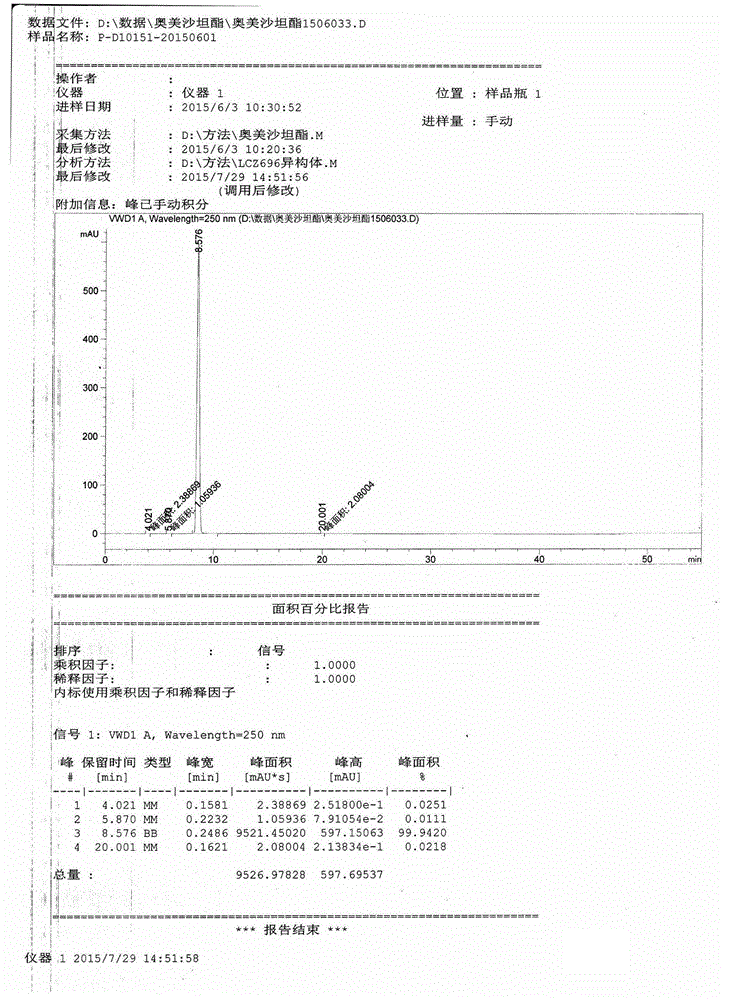
[0023] Put 10 g of crude olmesartan medoxomil and 80 g of methanol into the reaction flask, heat under reflux and stir to dissolve. After dissolution is completed, the solution is cooled to 40±5°C. After cooling down, add 160g petroleum ether dropwise to the bottle. After the dropwise addition is completed, the temperature in the bottle is reduced to 10°C, stirred for 2 hours to crystallize, filtered, and vacuum dried to obtain olmesartan medoxomil 8.5kg, yield 85.0%, HPLC purity 99.93 %.
Embodiment 2
[0025] Put 10g olmesartan medoxomil and 100g acetone into the reaction flask, heat under reflux and stir to dissolve. After the dissolution is completed, the solution is cooled to 30±5°C. After cooling, add 100g of isopropyl ether dropwise to the bottle. After the dropping, the temperature in the bottle is reduced to -10°C, stirred for 2 hours to crystallize, filtered and dried in vacuum to obtain 8.9g of Olmesartan medoxomil with a yield of 89.0%. HPLC The purity is 99.89%.
Embodiment 3
[0027] Put 10g of crude olmesartan medoxomil and 100g of acetonitrile into the reaction flask, heat to reflux and stir to dissolve, after dissolving, cool the solution to 30±5°C. After cooling down, add 200g methyl tert-butyl ether dropwise to the bottle. After the dropwise addition is complete, reduce the temperature in the bottle to 0°C, stir and crystallize for 2 hours, filter, and vacuum dry to obtain olmesartan medoxomil with a yield of 92.0%. , HPLC purity 99.90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com