Application of hyperin to treatment of ulcerative colitis
A technology for ulcerative colitis and hyperin, which is applied in the field of medicine, can solve the problems of many side effects, poor curative effect, and difficult long-term application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Hyperoside Anti-ulcerative Colitis Activity Test
[0021] 1. Materials
[0022] 1.1 Animals Wistar rats, weighing 180-200 g, half male and half male, were purchased from the Experimental Animal Research Center of Third Military Medical University.
[0023] 1.2 The drug hyperoside (Hyp) was purchased from Nanjing Zelang Pharmaceutical Technology Co., Ltd.
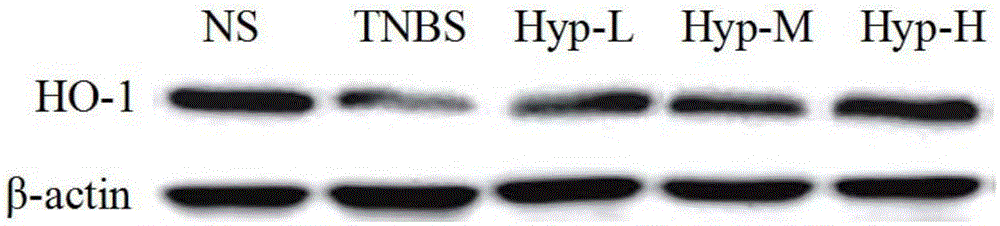
[0024] 1.3 Reagent 2,4,6-Trinitrobenzenesulfonic acid solution (2,4,6-Trinitrobenzenesulfonic acid solution, TNBS) aqueous solution was purchased from Sigma Company (5% (w / v); myeloperoxidase (MPO), propane Aldehyde (MDA), glutathione (GSH), superoxide dismutase (SOD) and Coomassie Leucine blue protein kits were purchased from Nanjing Jiancheng Bioengineering Institute; heme oxidase-1 (HO-1) antibody Purchased from CST Corporation.
[0025] 2. Method
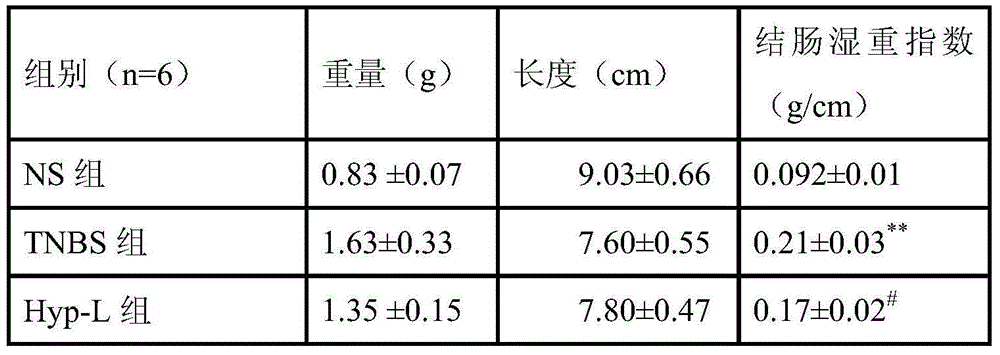
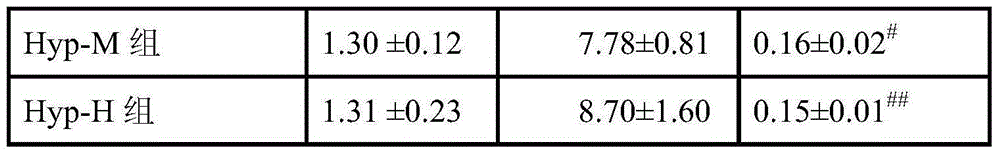
[0026] 2.1 Grouping Rats were randomly divided into 5 groups according to body weight, 6 in each group, respectively normal control group (NS), model cont...
Embodiment 2
[0051] The preparation of embodiment 2 hyperoside tablets
[0052] Hyperin 200g, add starch 200g, microcrystalline cellulose 60g, magnesium stearate 2g, mix evenly, granulate, dry, sieve and compress into tablets (1000 pieces), to obtain hyperin tablets .
Embodiment 3
[0053] The preparation of embodiment 3 hyperin capsules
[0054] Hyperin 300g, add starch 300g, magnesium stearate 2g, mix well, granulate, dry, pack into capsules (1000) after sieving, obtain the capsule of hyperin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com