Astragalus extract FQR-8 and application of astragalus extract FQR-8 in tumor cell immunotherapy
A technology of FQR-8 and Astragalus extracts, applied in animal cells, vertebrate cells, anti-tumor drugs, etc., to achieve the effects of prolonging tumor-bearing survival, increasing proliferation and reducing tumor load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] "Example 1" preparation of Astragalus extract FQR-8
[0034] Use a granulator to crush the crude drug of Astragalus membranaceus, fry it into cooked Astragalus membranaceus, add an appropriate amount of deionized water, make a slurry, add cellulase (Sinopharm Chemical Reagent Co., Ltd.) for 30 minutes, inactivate it, centrifuge at a high speed, and take the supernatant solution, and the supernatant was concentrated to make a concentrated solution, which was centrifuged again. Add ethanol to the supernatant, precipitate overnight at 4°C and centrifuge to obtain the precipitate. After the precipitate is dissolved with deionized water, it is added to the upper end of the pretreated macroporous adsorption resin XAD-16 column at a certain flow rate for adsorption. First wash slowly with deionized water to remove non-polar or water-soluble highly polar impurities such as polysaccharides or inorganic salts remaining on the surface or inside of the resin, and then use 20% meth...
Embodiment 2
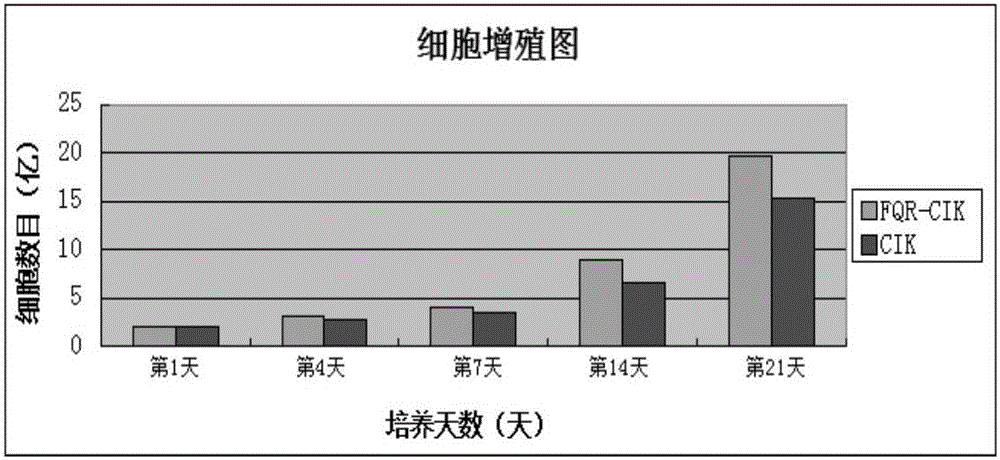
[0035] "Example 2" Isolation, induction and cultivation of immune cells DC-CIK and CIK
[0036] Patient preparation: According to the indications and contraindications of immune cell DC-CIK and CIK treatment, select tumor patients who are suitable for this treatment, inform the various relevant information and precautions involved in the treatment, and obtain the understanding and cooperation of patients and their families, Sign the informed consent form and perform routine blood tests;
[0037] Peripheral blood mobilization: 24 hours before blood collection, colony-stimulating factor (GM-CSF) 150ug should be injected subcutaneously;
[0038] Peripheral blood collection: patients should have a light diet before blood collection, aseptically extract 50ml of peripheral blood from patients with tumor diseases, anticoagulate with sodium heparin and mix well to avoid coagulation;
[0039] Tumor antigen acquisition: Centrifuge at room temperature at 2000 rpm for 10 minutes, collect...
Embodiment 3
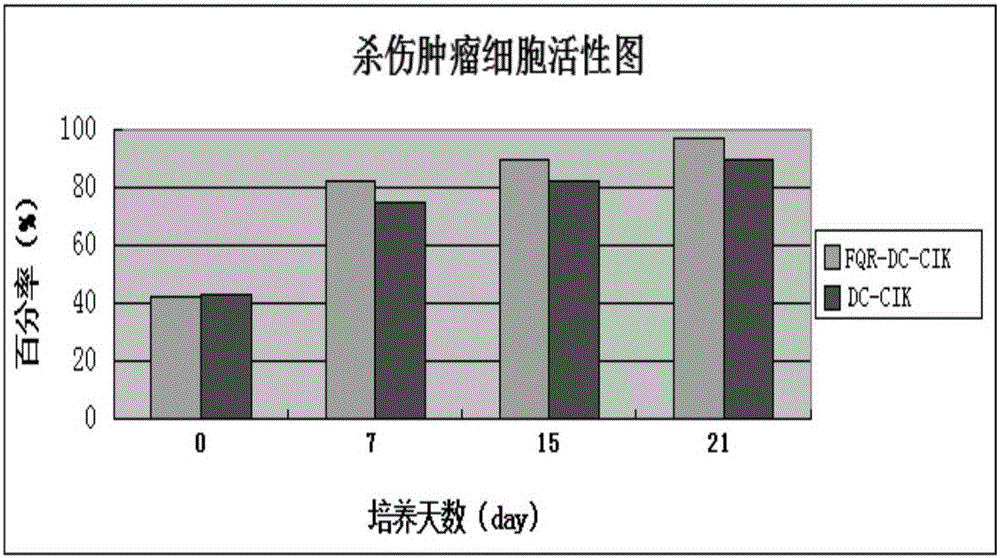
[0045] "Example 3" Induces the anti-tumor cell activity of activated FQR-DC-CIK cells
[0046] RPMI-1640 medium (Shanghai Kemin Biotechnology Co., Ltd.) adjusted the cell concentration to 1×10 5 / ml, respectively inoculated in 96-well cell culture plates, 100 μl per well, 8 wells per plate, a total of three plates;
[0047] Add 150ul 2×concentrated FQR-DC-CIK cells or 2×concentrated conventionally induced DC-CIK cells to each well, and at the same time, add NK cell sensitive cell line-K562 cell line (Basic Medicine, Chinese Academy of Medical Sciences) at a ratio of 1:10. Institute Cell Center). The first four wells of each plate are marked as the FQR-DC-CIK cell group, and the last four wells are marked as the conventionally induced DC-CIK cell group;
[0048] Set at 37°C, 5% CO 2 (V / V) co-cultivation in the incubator;
[0049] After culture, the OD570 tumor-killing activity was detected by MTT method;
[0050] Astragalus extract FQR-8 induced immune cell FQR-DC-CIK to k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com