Tumor targeted prodrug having endosome escaping function as well as nano preparation and preparation method of tumor targeted prodrug
An escape function and endosome technology, which is applied in the field of tumor-targeted prodrugs, can solve the problems of lack of endosome escape ability and inability to fully exert the drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
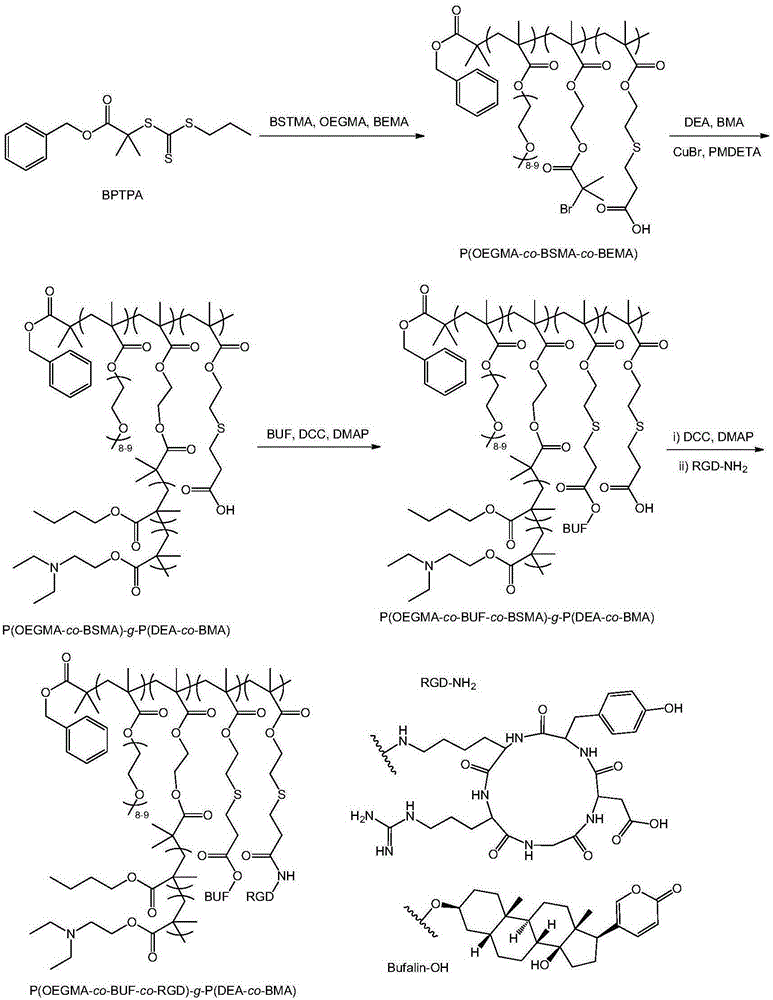
[0052] In the second aspect, the present invention provides a method for preparing the above-mentioned tumor-targeting prodrug with endosome escape function, and its synthesis route is shown in the attached figure 1 , including the following steps:
[0053] (1) Preparation of intermediate product P(OEGMA-co-BSMA-co-BEMA):
[0054] A certain amount of reversible addition-fragmentation chain transfer initiator BPTPA, oligoethylene glycol methacrylate OEGMA, monomer BSMA containing β-thiocarboxyl group, monomer BEMA containing atom transfer radical initiator, solvent and free Add the base initiator into the glass tube, freeze and thaw three times in vacuum and then seal the tube; then, react at 20-80°C for 0.5-40 hours to obtain the crude product P(OEGMA-co-BSMA-co-BEMA), and finally the The crude product P(OEGMA-co-BSMA-co-BEMA) was purified to obtain the P(OEGMA-co-BSMA-co-BEMA).
[0055] (2) Preparation of intermediate product P(OEGMA-co-BSMA)-g-P(DEA-co-BMA):
[0056]Add a...
Embodiment 1
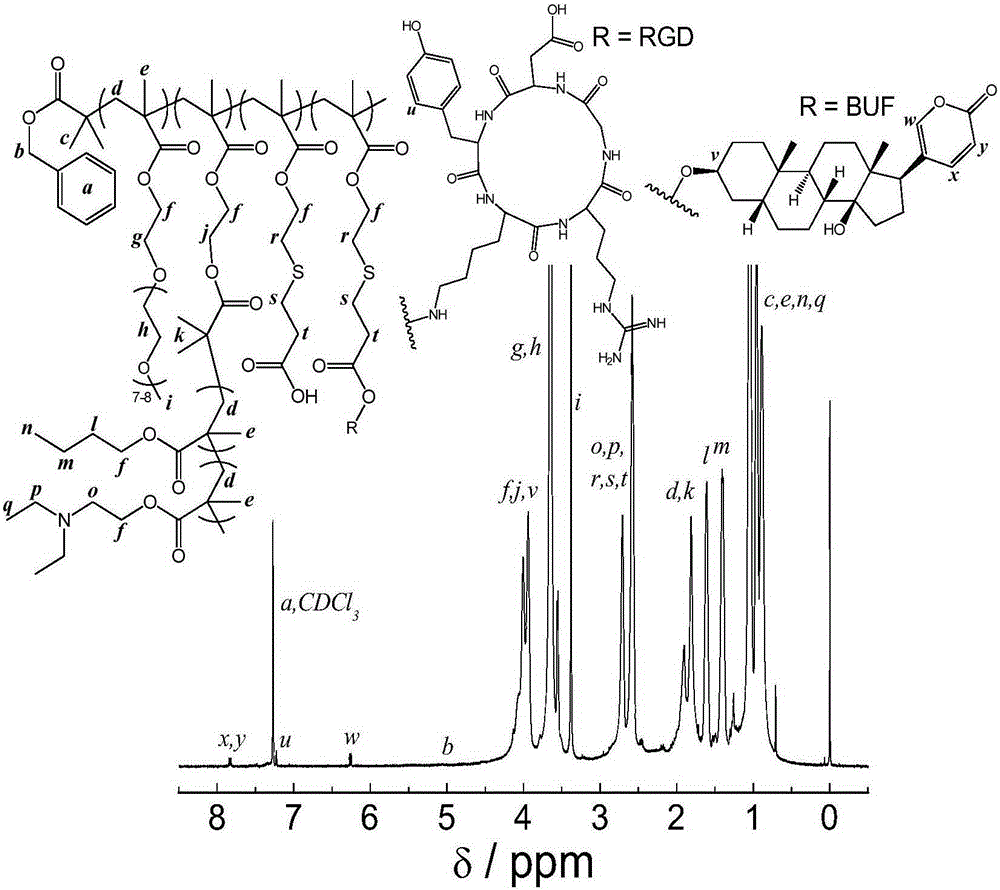
[0083] cRGD-modified poly(oligoethylene glycol methacrylate-co-bufalin-co-RGD)-g-poly(N,N-diethylaminoethyl methacrylate- co-n-butyl methacrylate) tumor targeting prodrug, namely the preparation of P(OEGMA-co-BUF-co-RGD)-g-P(DEA-co-BMA):
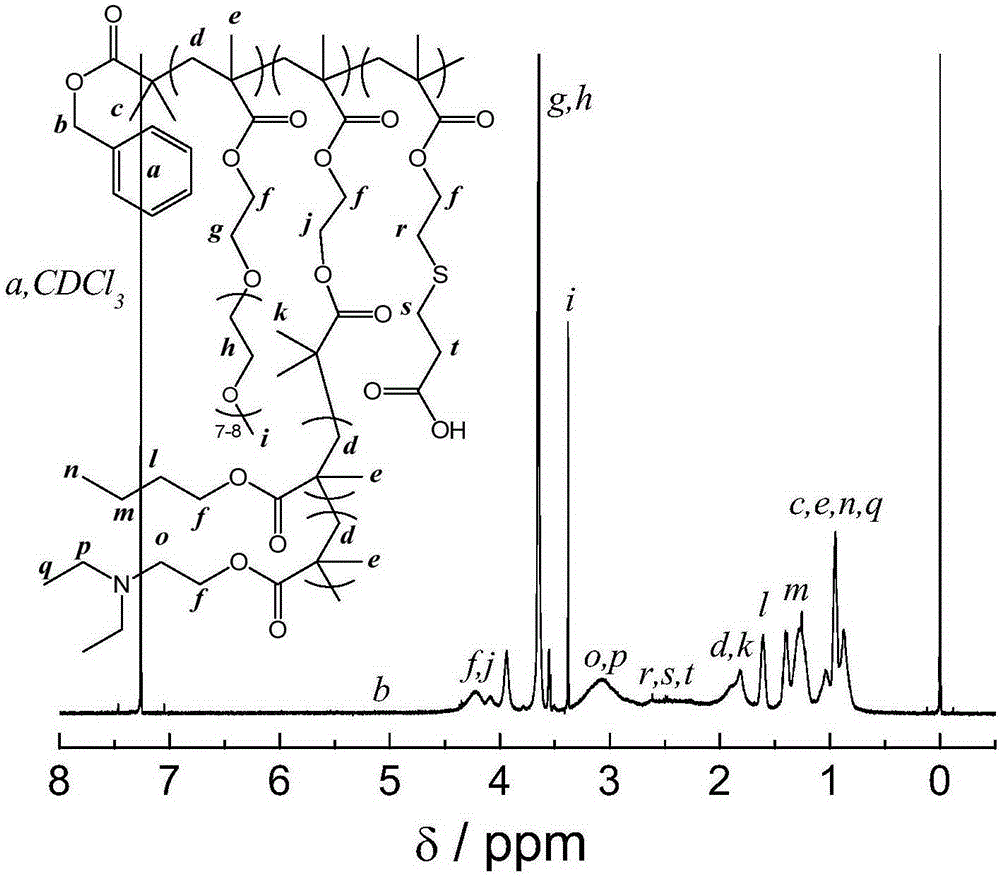
[0084] (1) Preparation of P(OEGMA-co-BSMA-co-BEMA): BPTPA (33mg0.1mmol), OEGMA (3.0g, 6mmol), BSMA (686mg, 2.5mmol), BEMA (140mg, 0.5mmol) and AIBN (1.6 mg) was added to a glass tube, and 10 mL of dioxane was added, and the tube was sealed after vacuum freezing and thawing three times. Thereafter, the reaction was carried out at 70° C. for 5 hours. The crude product obtained from the reaction was dialyzed in water for 24 hours to remove impurities, and after freeze-drying, the P(OEGMA-co-BSMA-co-BEMA) was obtained. The molecular weight M of the obtained P (OEGMA-co-BSMA-co-BEMA) determined by GPC n =30,300, molecular weight distribution M w / M n = 1.07.
[0085](2) Preparation of P(OEGMA-co-BSMA)-g-P(DEA-co-BMA): P(OEGMA-co-BSMA-co-BEM...
Embodiment 2
[0089] Preparation of P(OEGMA-co-BUF-co-RGD)-g-P(DEA-co-BMA) nanoparticle solution:
[0090] Dissolve 10 mg of P(OEGMA-co-BUF-co-RGD)-g-P(DEA-co-BMA) in 1 mL of DMSO, drop the solution into 9 mL of stirring water, and then put the solution into a dialysis bag (molecular weight cut-off of 7,000 Da ) in dialysis for 24 hours to remove the organic solvent to obtain a nanoparticle solution. Its particle size is about 148.4nm (±0.65) (see Figure 4 ), potential -7.6mV (±0.37). Cytotoxicity experiments show that its killing effect on various cancer cells is significantly better than that of free bufolin. Such as Figure 5 As shown, the killing effect of targeted nano-drugs on colorectal cancer cell line LoVo, its IC50 is much lower than that of free bufalin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Mwco | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com