A novel universal matrix vaccine adjuvant and its preparation method and use
A matrix adjuvant and adjuvant technology, applied to medical preparations containing active ingredients, antiviral agents, pharmaceutical formulas, etc., can solve problems such as tissue necrosis, severe side effects, and poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
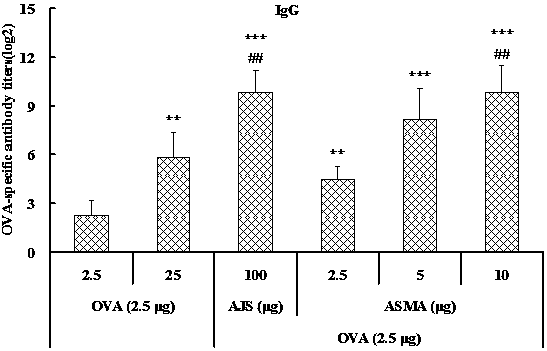
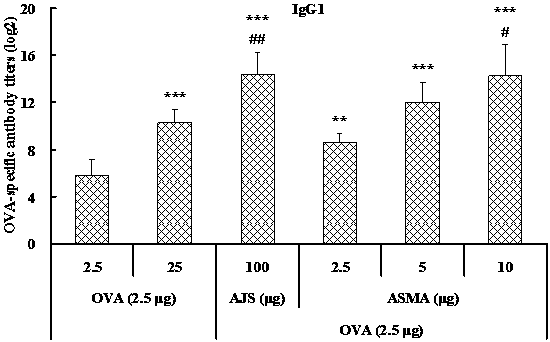
[0034] Example 1: Preparation of Albizia Julibis Saponin Matrix Adjuvant (ASMA) by Dialysis
[0035] 1. Preparation of 5% Albizia Julibis Saponin Solution: Dissolve Albizia Julibis Saponin in distilled water to make a 50 mg / ml solution, filter and sterilize, and set aside.
[0036] 2. Preparation of 1% cholesterol / lecithin solution: take non-ionic surfactant Mega-10, dissolve in double distilled water to make a 20% solution; then weigh lecithin and cholesterol, dissolve in 20% Mega- 10 aqueous solution, make each ml contain 10 mg of lecithin and 10 mg of cholesterol, filter and sterilize, and set aside.
[0037] 3. Orthogonal experimental design: using L 9 (3 4 ) orthogonal table to investigate the prescription and process conditions. The factors and level design of the orthogonal experiment are shown in Table 1, and the experimental arrangement is shown in Table 2.
[0038] Table 1 Orthogonal test factor level table
[0039]
[0040] Table 2 Orthogonal test arrangemen...
Embodiment 2
[0046] Example 2: Preparation of Albizabis saponin matrix adjuvant (ASMA) by ultrafiltration
[0047] 1. Preparation of 5% Albizia Julibis Saponin Solution: Dissolve Albizia Julibis Saponin in distilled water to make a 50 mg / ml solution, filter and sterilize, and set aside.
[0048] 2. Preparation of 1% cholesterol / lecithin solution: take non-ionic surfactant Mega-10, dissolve in double distilled water to make a 20% solution; then weigh lecithin and cholesterol, dissolve in 20% Mega- 10 aqueous solution, make each ml contain 10 mg of lecithin and 10 mg of cholesterol, filter and sterilize, and set aside.
[0049] 3. Orthogonal experimental design: using L 9 (3 4 ) orthogonal table to investigate the prescription and process conditions. The factors and level design of the orthogonal experiment are shown in Table 1, and the experimental arrangement is shown in Table 2.
[0050] 4. Sample preparation is enlarged 5 times according to the volume in Table 3, add 1% cholesterol / l...
Embodiment 3
[0052] Example 3: Preparation of Albizia Julibrissin Matrix Adjuvant (ASMA) from Lecithin from Different Sources
[0053] 1. Preparation of 5% Albizia Julibis Saponin Solution: Dissolve Albizia Julibis Saponin in distilled water to make a 50 mg / ml solution, filter and sterilize, and set aside.
[0054] 2. Preparation of 1% cholesterol / lecithin solution: prepare a 20% aqueous solution with non-ionic surfactant Mega-10 or octyl glucoside. Then cholesterol and phosphatidylcholine, soybean lecithin or egg yolk lecithin were dissolved in 20% Mega-10 aqueous solution to obtain three groups of samples, containing 10 mg / ml each of phosphatidylcholine and cholesterol, and soybean lecithin and cholesterol 10 mg / ml each, or yolk lecithin and cholesterol 10 mg / ml each, sterilized by filtration and set aside.
[0055] 3. Sample preparation Take 0.3 ml each of the 1% cholesterol / lecithin solutions prepared from the above three different sources of lecithin, add 0.6 ml of PBS buffer solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com