Polysaccharide with immunologic adjuvant effect, preparation method and application thereof
An immune adjuvant, polysaccharide technology, applied in biochemical equipment and methods, medical preparations containing active ingredients, vaccines, etc., can solve problems such as toxic side effects and safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Preparation of crocus petal polysaccharide
[0047] 1. Preparation of total polysaccharides from crocus petals
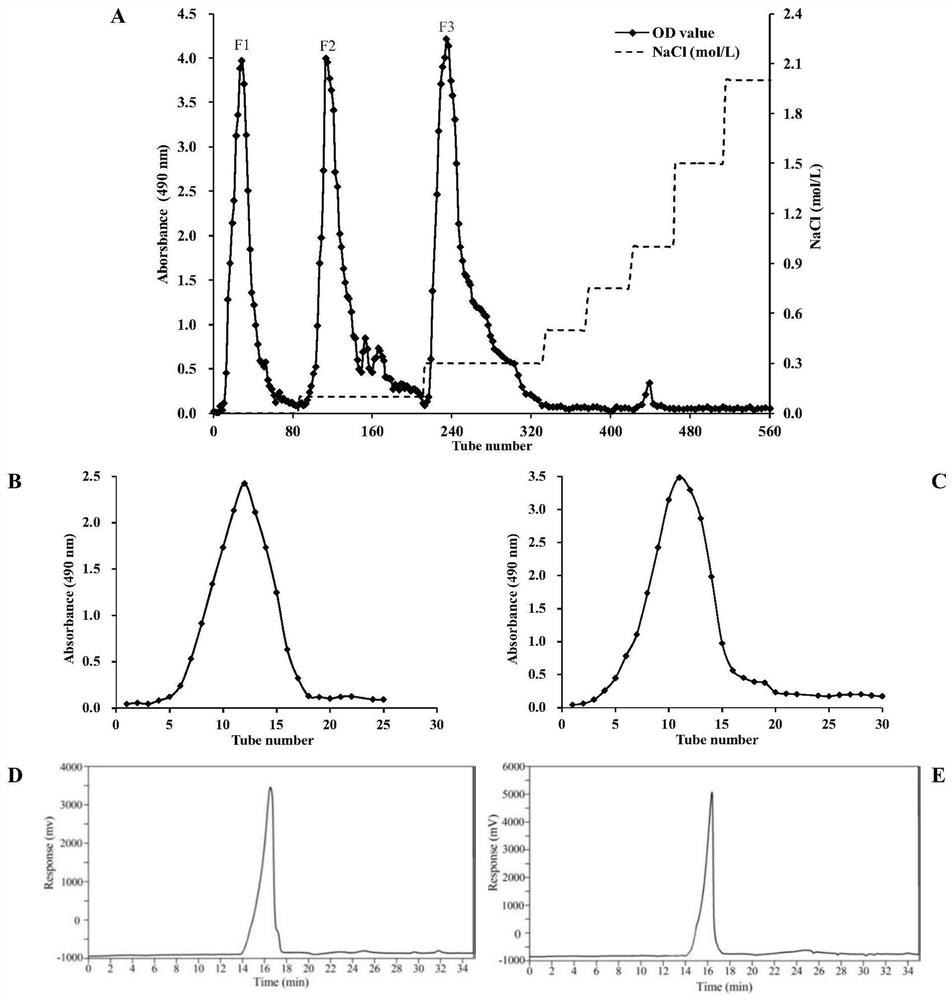
[0048] Add 400 g of saffron petals, add 40 times of water, heat and reflux for extraction for 2 hours, filter, and continue extracting the dregs twice with water and reflux. Combine the filtrates, recover them under reduced pressure to 600 mL, place them at room temperature, add 4 times the volume of 95% ethanol with a mass concentration, mix well, and let stand at 4°C for 24 hours to precipitate. Fast filter paper was filtered under reduced pressure, and the precipitate was collected, washed three times with ethanol, acetone and petroleum ether successively. The precipitate was dissolved with distilled water and the volume was adjusted to 300mL, 120mL of papain solution with a concentration of 10mg / mL was added, mixed evenly, and enzymatically hydrolyzed at a constant temperature of 60°C for 1h. Then add 105 mL of Sevage reagent, stir at room...
Embodiment 2
[0053] Embodiment 2: Molecular weight and uniformity determination of saffron petal polysaccharide A and saffron petal polysaccharide B
[0054] Saffron petal polysaccharide A (11 mg) and saffron petal polysaccharide B (10 mg) were weighed respectively, dissolved in water to make a solution with a concentration of 1 mg / mL, and filtered through a 0.45 μm filter membrane to obtain the sample solution. Another standard polysaccharide with different molecular weight (Mw) was taken to prepare a series of standard polysaccharide solutions. Using Angilent 1260 liquid chromatography system, Shodex ks805 chromatographic column (8.0 × 300mm), H 2 O is the mobile phase, the flow rate is 1mL / min; the column temperature is 35°C, the injection volume is 1μL, and the differential detector is used for measurement. With logMW as the ordinate and retention time as the abscissa, draw a standard curve to obtain the regression equation: log MW=- 0.2839X+10.749(R 2 = 0.9988). The result shows; T...
Embodiment 3
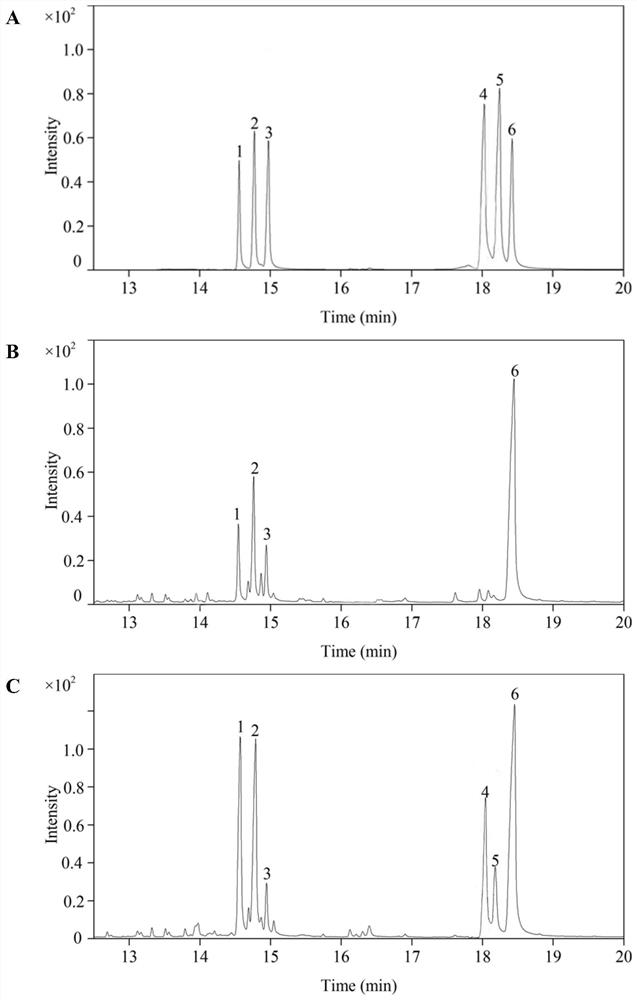
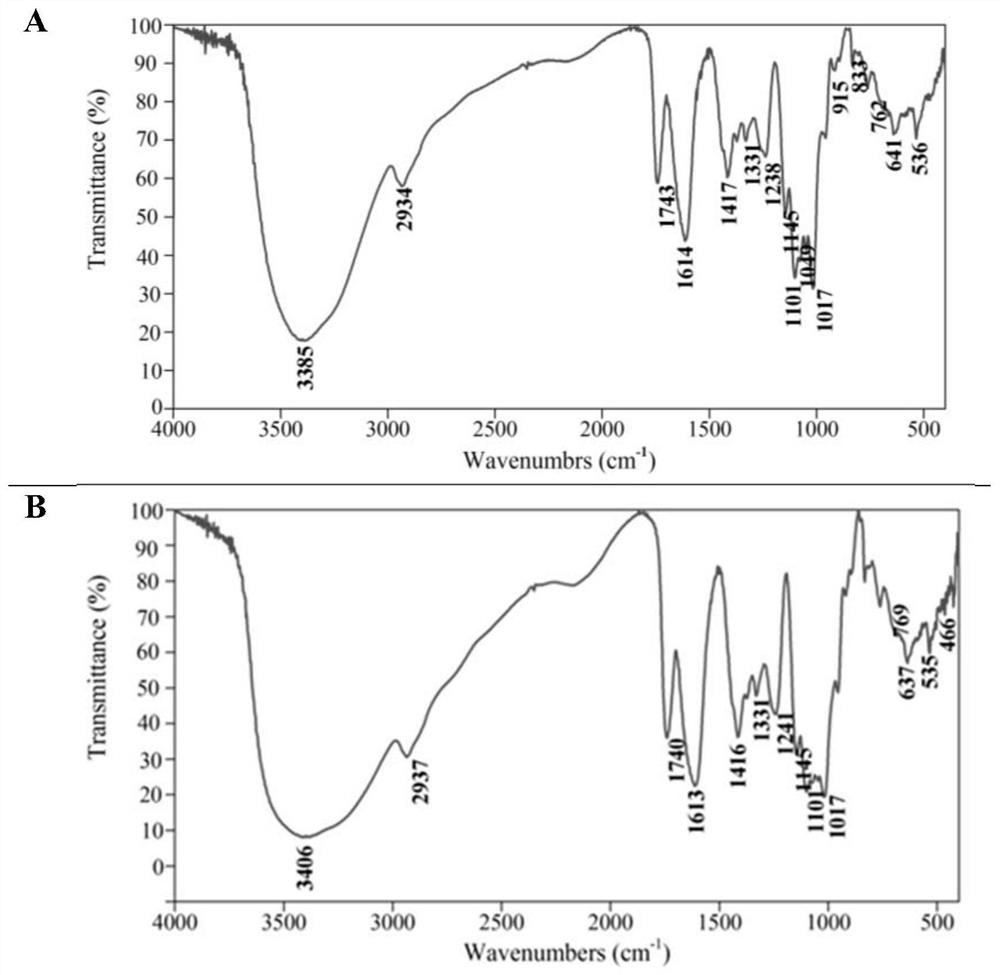
[0055] Example 3: Structural characterization of saffron petal polysaccharide A and saffron petal polysaccharide B
[0056] 1. Monosaccharide composition and molar ratio of crocus petal polysaccharide A and crocus petal polysaccharide B (GC-MS analysis)
[0057] Weigh about 10 mg of saffron petal polysaccharide A and saffron petal polysaccharide B, and hydrolyze with 2M trifluoroacetic acid at 110°C for 5 hours; evaporate the hydrolyzate to dryness under reduced pressure, add NaBH 4 , reduced at 60°C for 5h; added acetic acid to consume excess NaBH4; added 10% acetic acid in methanol, and evaporated to dryness repeatedly to obtain the reduced product. Acetylation of crocus petal polysaccharide A, crocus petal polysaccharide B, rhamnose, arabinose, xylose, mannose, glucose and glucose reduction products with pyridine-acetic anhydride (1:1) under acidic conditions , the acetylated product was redissolved in chloroform, filtered through a 0.22 μm microporous membrane, and analyz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com