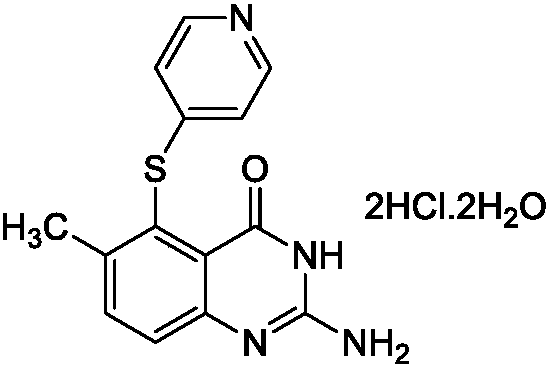
Preparation method of lolatrexex hydrochloride freeze-dried powder injection
A technology of lolatrexel hydrochloride and freeze-dried powder injection, which is applied in the field of preparation of lolatrexel hydrochloride freeze-dried powder injection, can solve the problems of impractical clinical application, unsatisfactory drug storage and transportation, and high content of related substances. Achieve the effect of facilitating industrial production, ensuring effectiveness and safety, and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Prescription of lolatrexel hydrochloride freeze-dried powder injection
[0033] Specification: 500mg / bottle
[0034]
[0035] 2. Preparation method
[0036] 1) Weigh the prescribed amount of lolatrexex hydrochloride and mannitol respectively, add about 80% of the prescribed amount of water for injection (80°C), stir and dissolve, add 1M sodium hydroxide solution to adjust the pH value to 2.0, add water for injection to 4000g.
[0037] 2) Filter and sterilize the drug solution obtained in step (1) with a microporous membrane to obtain a fine filtrate, which is subpackaged, filled with 4 g of the drug solution in each vial, and half-covered with a rubber stopper.
[0038] 3) Freeze-dry the fine filtrate contained in the vial:
[0039] Start the freeze dryer, and after pre-freezing at -30°C for 2 hours, start the vacuum pump, raise the shelf temperature to -15°C for sublimation drying for 20 hours, then raise the temperature to 5°C and dry for 4 hours; After the ...
Embodiment 2-5
[0054] The lolatrexex hydrochloride freeze-dried powder injection was prepared according to the method of Example 1, with the difference that the pH value in step 1) was adjusted to 1.8, 1.9, 2.1, and 2.2 with 1M hydrochloric acid and 1M sodium hydroxide solution, respectively.
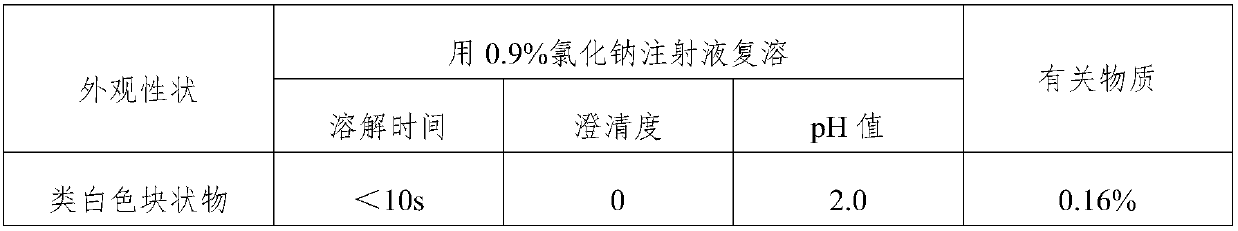
[0055] The freeze-dried powder injections obtained in Examples 2-5 were subjected to a comparative study on appearance properties, reconstitution and clarity, and compared with Control Example 1 prepared by the prior art. The results are detailed in Table 3.
[0056] The detection result of table 3 embodiment 1-5 lyophilized powder injection
[0057]
[0058] When the pH range of the drug solution before freeze-drying is controlled between 1.8 and 2.2, the clarification values of the prepared freeze-dried powder after reconstitution are all less than 1, which is almost clear and can meet the requirements of clinical use. When the pH value of the drug solution before freeze-drying is controlled b...
Embodiment 6-11
[0060] According to the method of Example 1, lolatrexex hydrochloride freeze-dried powder injection was prepared, the difference is that the freeze-drying process in step 3) adopts different freeze-drying curves respectively, and compares it with the control example 1 prepared by the prior art, and the results are shown in the table 4.
[0061] The detection result of table 4 embodiment 6-11 freeze-dried powder injection
[0062]
[0063] According to the appearance, reconstitution speed, clarity and test results of related substances, the freeze-drying process parameters of lolatrexex hydrochloride for injection are set as follows: -30~-20℃ for 2-4 hours, -15℃~-10 Sublimation at ℃ for 10-20 hours, followed by secondary drying at 5℃-15℃ for 0-4 hours, can obtain samples with good appearance, fast redissolving, clear liquid medicine and lower content of related substances.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com