Pharmaceutical ranitidine hydrochloride composition for treating digestive diseases
A technology for ranitidine hydrochloride and digestive diseases, which is applied in the field of drug ranitidine hydrochloride composition for treating digestive diseases, can solve problems such as drug safety impact, toxic and side effects, and achieves low content of insoluble particles and stable Good performance and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
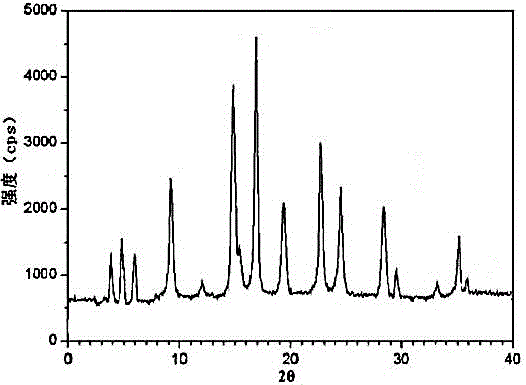
[0024] Example 1: Preparation of ranitidine hydrochloride crystals
[0025] 1) Prepare mixed solution A by mixing N-methylpyrrolidone and water at a volume ratio of 1:5;
[0026] 2) Take the ranitidine hydrochloride raw material, add the mixed solution A prepared in step 1), wherein the ratio of the volume of the mixed solution A to the mass of ranitidine hydrochloride is 15ml:1g, heat to 35°C, After stirring to dissolve all the solution, add 0.1% g / ml activated carbon to decolorize and filter to obtain a clear solution;
[0027] 3) Prepare mixed solution B with acetone and ethylene glycol at a volume ratio of 4:1.5;
[0028] 4) At room temperature, add mixed solution B to the clear solution obtained in step 2) under an ultrasonic field with a power of 0.6KW, where the amount of mixed solution B added is 6 times the volume of mixed solution A, and turn off the ultrasonic field after adding , cooled to -10°C, allowed to stand for 2 hours, crystals were precipitated, and dri...
Embodiment 2
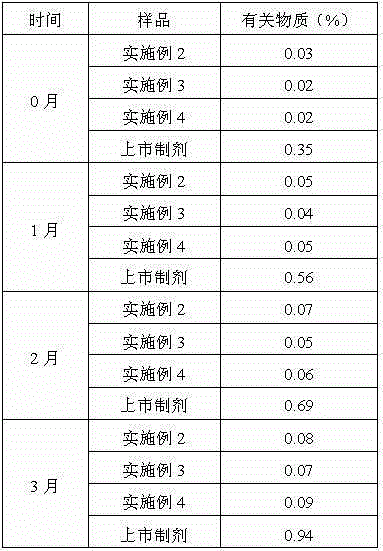
[0030] Example 2: Preparation of ranitidine hydrochloride composition
[0031] The composition is: 1 part by weight of ranitidine hydrochloride crystal prepared by the present invention, and 0.01 part by weight of arginine.
[0032] The preparation method is:
[0033] (1) Weigh ranitidine hydrochloride crystals and arginine in proportion and mix them thoroughly;
[0034] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0035] Example 3: Preparation of ranitidine hydrochloride composition
[0036] The composition is: 1 part by weight of ranitidine hydrochloride crystal prepared by the present invention, and 0.02 part by weight of arginine.
[0037] The preparation method is:
[0038] (1) Weigh ranitidine hydrochloride crystals and arginine in proportion and mix them thoroughly;
[0039] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com